Erythrocyte morphology changes not only by primary hematological diseases but also by systemic inflammation, ineffective erythropoiesis and nutritional deficiencies. Red blood cell distribution width (RDW) is a parameter reflecting erythrocyte morphology. We aimed to investigate the relationship of RDW with chronic obstructive pulmonary disease (COPD) stages, BODE index and survival in COPD patients.
MethodsMedical records of 385 COPD patients between July 2004 and November 2005 were studied retrospectively. Demographic features, BODE index factors and oxygen saturation were recorded. Survival analysis of all patients by 2014 was performed. Measured RDW values at the time of the inclusion were evaluated.
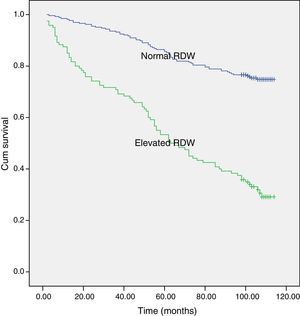
ResultsMean age of the patients was 65.6±9.6 years. Distribution of the COPD stages of the patients were stage 1: 16%, stage 2: 52%, stage 3: 26%, stage 4: 6%. RDW was found significantly different between stages. The highest RDW was observed in the very severe stage (p<0.001). Median of BODE index was 1 (0–3). As the BODE index increased RDW also increased (p<0.001). When the patients were grouped according to the laboratory upper limit of RDW, survival rate was 31% in the RDW >14.3% group and 75% in the RDW <14.3% group.
ConclusionThe variability in the size of circulating erythrocytes increases as the COPD severity progresses. Therefore, a simple and noninvasive test, such as RDW, might be used as a biomarker in the evaluation of the severity of COPD. At the same time, there seems to be a correlation between the survival of COPD patients and RDW.
Chronic obstructive pulmonary disease (COPD) is defined as a preventable and treatable disease characterized by usually progressive persistent airflow limitation that is associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases.1 However, it is still the fifth leading cause of death and estimated to be the third in 2030 worldwide.2 Currently, COPD has been accepted as a component of systemic inflammatory syndrome and the mortality mostly depends on cardiovascular diseases rather than respiratory failure.3 Possible explanations for the high cardiovascular morbidity and mortality observed in COPD patients are high smoking prevalence, diet and sedentary life style as well as systemic inflammation due to oxidative stress and chronic hypoxia.4,5
The red cell distribution width (RDW) is a routine laboratory parameter that indicates the variability in the size of circulating erythrocytes. The main area which the RDW is used is the differential diagnosis of microcytic anemia. It has been defined as a prognostic tool in different clinical settings such as pulmonary arterial hypertension, congestive heart failure and coronary heart disease.6–8 It has also been reported as a powerful predictor of mortality in general population and older adults.9,10 Increased RDW values have been reported to be related with underlying chronic inflammation which promotes red blood cell membrane deformability and changes in erythropoiesis.11
Accordingly, we hypothesized that systemic inflammation may be the common link between increased RDW values and mortality in patients with COPD. Therefore we aimed to study the relationship between RDW and airflow limitation severity stages, BODE index and survival in COPD patients.
MethodsWe conducted a retrospective cohort study in patients diagnosed as COPD by global initiative for chronic obstructive lung disease (GOLD) criteria between July 2004 and November 2005 to observe the 9-year survival rate.1 The inclusion criteria was: being on stable state (no history of hospitalization or admission to an emergency department for at least 8 weeks), absence of cancer history, connective tissue disorders, inflammatory bowel disease, hematological system diseases, blood transfusion and anti-inflammatory drug (systemic steroids, immunosuppressive drugs) usage in the last 2 months. In January 2014 we searched for the survival of these patients in the registry to evaluate 9-year survival rate. We used the hospital automation software which was integrated with the national population system to determine the deaths.
Demographic features and medical history including comorbid diseases such as hypertension, diabetes mellitus, cardiovascular disease and smoking status of the study population were recorded. Six-minute walk test (6MWT), pulmonary function tests (PFT), BODE index and complete blood count results at the time of the inclusion (July 2004 and November 2005) were also collected from the medical records of the patients.
It would be better to use the composite COPD assessment index recommended by GOLD since 2011. That was not possible because at the time of the study, dyspnea scores and the number of exacerbations in the previous year were not accessible.
Pulmonary function testsPFTs were performed with Jaeger Master Screen Pneumo V452İ device by a single technician between July 2004 and November 2005. The best test among the three consecutive ones was accepted. FEV1 (Forced expiratory volume in 1s), FVC (Forced vital capacity), FEV1/FVC (percentage of FVC expelled in the first second of a forced expiration) were measured according to American Thoracic Society criteria.12 The airflow limitation severity staging was done according to GOLD 2015.1
Laboratory measurementsBlood samples obtained between July 2004 and November 2005 were evaluated. RDW normal range was between 11.8% and 14.3% in our laboratory.
Six-minute walk test6MWT was performed in a corridor of 35m at the time of diagnosis. Patients were motivated to walk as fast as they could. Oxygen saturation was measured before and after the test and the distance walked was recorded.13
BODE indexBODE index was calculated according to body mass index (BMI), FEV1, 6MWT, modified medical research council dyspnea scale (MMRC) at the time of diagnosis.14
Oxygen saturationOxygen saturation was measured by pulse oxymeter at the time of diagnosis (Plusmed).
Statistical analysesData were analyzed by SPSS 15.0 package program. The frequencies of the results were expressed according to the dispersion properties of data. Variables were compared by unpaired t tests for continuous data and Mann Whitney U-test for nonparametric data. Chi-square analysis was used for categorical data. Spearman and Pearson correlation tests were used for correlation analysis. A p value, <0.05 was considered significant.
Survival analysis was performed thereafter grouping patients according to the laboratory upper limit of RDW. The patients’ inclusion date in the study was considered as the first day. The last check date or the discharge date was considered as the last day on survival analysis. Kaplan–Meier survival analysis was performed for univariate survival analysis. Multivariate analysis for survival rates were further performed by Cox proportional hazards regression to adjust for age, comorbid diseases, FEV1 and 6MWT.
Ethics approval was obtained from Dokuz Eylul University Faculty of Medicine Local Ethics Committee and we have signed informed consent from all patients.
ResultsThree hundred eighty five COPD patients were included in the analysis. Mean age of the study population was 65.6±9.6 years. Most of the patients were males, ex-smokers and had comorbidities (Table 1).
Demographic characteristics of the study population.
| n (%) | |
|---|---|
| Gender | |
| Male | 338 (88) |
| Female | 47 (12) |
| GOLD stages | |
| Stage 1 | 64 (16) |
| Stage 2 | 200 (52) |
| Stage 3 | 99 (26) |
| Stage 4 | 22 (6) |
| Smoking status | |
| Non-smoker | 50 (13) |
| Smoker | 125 (32) |
| Ex smoker | 210 (55) |
| Comorbidity | |
| Yes | 226 (59) |
| CVSD | 214 (56) |
| DM | 33 (9) |
| Others | 122 (32) |
| No | 159 (41) |
| Mortality cause | |
| Cancer | 28 (9) |
| COPD | 30 (8) |
| Pneumonia | 20 (5) |
| CVSD | 31 (10) |
| Renal failure | 5 (1) |
| Neurologic disorder | 2 (1) |
| BODE index (median/min-max) | 1.0 (0.0–10.0) |
COPD: chronic obstructive pulmonary disease, CVS: cardiovascular system disorders, DM: diabetes mellitus, Others: neuromuscular system disorders and renal diseases, BODE: body mass index, airflow obstruction, dyspnea and exercise capacity.
Distribution of the COPD stages of the patients were stage 1: 16%, stage 2: 52%, stage 3: 26%, stage 4: 6%. Median of the BODE index was 1 (0–3). Mean RDW levels were found to be increasing with the severity of COPD (Table 2).
RDW was inversely correlated with the 6min walk test, pulmonary functional parameters and oxygen saturation. There was also a mild association with age, GOLD stage and a moderate correlation with BODE index (Table 3).
Correlation of RDW with functional and demographic parameters.
| RDW correlation | r | p |
|---|---|---|
| 6MWT | −0.279 | <0.001 |
| %FEV1 | −0.290 | <0.001 |
| %FVC | −0.285 | <0.001 |
| FEV1/FVC | −0.206 | <0.001 |
| %FEF 25–75 | −0.303 | <0.001 |
| %PEF | −0.227 | <0.001 |
| Oxygen saturation | −0.260 | <0.001 |
| BMI | −0.059 | 0.247 |
| Age | 0.182 | <0.001 |
| BODE index | 0.407 | <0.001 |
| GOLD stage | 0.403 | <0.001 |
6MWT: 6min walk test, FEV1: forced expiratory volume in 1s, FVC: forced vital capacity, FEF 25–75: forced expiratory flow rate 25–75%, PEF: peak expiratory flow, BMI: body mass index, RDW: red cell distribution width.
Pulmonary functional parameters, 6MWT distance and oxygen saturation were lower for patients with an elevated RDW, while age, smoking intensity and BODE index were higher (Table 4).
Functional and demographic parameters of the study population according to upper limit of RDW values.
| All patients | RDW≤14.3 | RDW>14.3 | p | |
|---|---|---|---|---|
| Age | 65.6± | 64.4 | 68.0 | =0.001 |
| FEV1 (L) | 1.66 | 1.80 | 1.34 | <0.001 |
| %FEV1 | 60.2 | 64.6 | 50.5 | <0.001 |
| FVC (L) | 2.86 | 3.04 | 2.45 | <0.001 |
| %FVC | 80.8 | 85.1 | 71.2 | <0.001 |
| FEV1/FVC | 57.2 | 58.7 | 53.9 | <0.001 |
| PEF (L) | 4.79 | 5.1 | 4.1 | <0.001 |
| %FEF 25/75 | 26.1 | 28.7 | 20.3 | <0.001 |
| Smoking (packyears) | 56.0 | 52.5 | 63.3 | =0.005 |
| Smoking beginning age | 17.8 | 18.2 | 16.8 | =0.02 |
| BMI | 25.4 | 25.6 | 24.9 | =0.09 |
| 6MWT (meters) | 435 | 453 | 393 | <0.001 |
| Oxygen saturation (%) | 96.0 | 96.4 | 95.1 | <0.001 |
| BODE index | 1.83 | 1.32 | 2.98 | <0.001 |
| Survival (months) | ||||
| Alive | 236 | n: 199 75% | n: 37 31% | <0.001 |
| Exitus | 149 | n: 66 25% | n: 83 69% | |
FEV1: forced expiratory volume in 1s, FVC: forced vital capacity, FEF 25–75: forced expiratory flow rate 25–75%, PEF: peak expiratory flow, BMI: body mass index, 6MWT: 6min walk test. BODE: body mass index, airflow obstruction, dyspnea and exercise capacity. Data were expressed as mean±standard deviation.
Median follow up time for the study population was 84.5 months (2–114 months). The overall 9-year survival rate of the study population was 61%. When the patients were divided into 2 groups according to RDW the 9-year survival rate was 75% for patients with normal RDW (≤14.3%) and 31% for patients with an elevated RDW (>14.3%) (p<0.001) (Fig. 1). On multivariate analysis, when adjusted for potential confounders, age and 6MWT were found to be associated with survival (Table 5).
Impact of RDW and potential confounders on survival.
| Odds ratio | 95% confidence interval | p value | |
|---|---|---|---|
| FEV1 | 0.529 | 0.375–0.747 | <0.001 |
| RDW | 1.222 | 1.153–1.295 | <0.001 |
| 6MWT | 0.998 | 0.997–1.000 | 0.007 |
| Age | 1.055 | 1.033–1.077 | <0.001 |
| Comorbidities | 1.430 | 1.004–2.036 | <0.01 |
FEV1: forced expiratory volume in 1s, RDW: red cell distribution width, 6MWT: 6min walk test.
COPD is a chronic respiratory disease with significant mortality and morbidity. Elevated levels of RDW have been reported to be related with cardiovascular mortality.4 Considering RDW as a mortality predictor, in this study we investigated the relationship between RDW and airflow limitation severity stages, BODE index and survival in COPD patients. We found increased RDW levels associated with COPD severity and also observed a higher mortality in patients with increased RDW levels.
A recent population based study reported a negative and independent association of RDW with lung function after eliminating the effects of potential confounders.15 The study could not determine any interaction between RDW and smoking and questioned the direct effects of RDW on pulmonary functions. However, in another study, smokers RDW levels were found to be higher than the ones associated with non-smokers.16 In another study investigating the prognostic usefulness of RDW in idiopathic pulmonary fibrosis, the authors found that the patients with lower FEV1 and lower diffusing capacity had higher RDW and concluded that RDW was an independent prognostic information at baseline and follow-up.17
The results of our study also demonstrated that pulmonary function test parameters were negatively associated with RDW. This finding was concordant with the high RDW levels we observed in severe COPD stages, especially in the very severe group. We also detected a relationship between smoking intensity and RDW levels, which might be due to hypoxemia related erythropoiesis. The correlation of the oxygen saturation and RDW levels also supports this hypothesis.
BODE index, which has been found to be related with mortality in COPD, was associated with RDW in our study. According to our knowledge, the relationship between RDW and exercise capacity in COPD patients has not been shown before. In this study elevated RDW levels were found significantly related with poor exercise capacity in COPD patients. There is a limited number of studies investigating the association of BODE index with systemic inflammatory markers other than RDW. A relation with C reactive protein and leptin has been reported while tumor necrosis factor alfa and interleukin 6 were found to be unrelated.18–20 In a recent study investigating the prognostic value of RDW in COPD, cardiac, respiratory, and hemotological status were evaluted and RDW was found to be correlated with C reactive protein, pulmonary arterial hypertension and right ventricular dysfunction.21 As we found increased RDW levels in severe COPD similarly, we think that RDW could also be considered as a prognostic systemic inflammatory marker in COPD.
The independent association between RDW and survival has been described for a broad spectrum of cardiovascular and pulmonary disease such as pulmonary hypertension, acute pulmonary embolism, idiopathic pulmonary fibrosis and even for COPD.6,17,21,22 The underlying mechanisms for the association between the RDW and mortality are currently unknown. Nutritional deficiencies, comorbid diseases, hematological aberrations and impaired renal functions occurring in the course of chronic cardiopulmonary diseases may result in subtle anemia, reflected by increased RDW.8,23–25 Elevated RDW values may also be associated with low grade inflammation that is present in chronic respiratory diseases. Inflammation induces changes in erythropoiesis, erythrocyte half-life, and erythrocyte membrane deformability.22 Accordingly, we observed a lower survival rate in COPD patients who have elevated RDW levels.
The limitations of the study include a relatively long period of observation of survival rates, as well as not discriminating respiratory system mortality from all-cause mortality due to lack of data. This might be related to the high rates of mortality we observed in our cohort of COPD patients at early stages of the disease. Another restriction is the lack of a control group with similar demographics but without COPD to better demonstrate the effect of the disease on RDW. It would also be better to use the composite COPD assessment index recommended by GOLD since 2011. That was not possible because at the time of the study, dyspnea scores and the number of exacerbations in the previous year were not accessible.
ConclusionIn conclusion, we demonstrated that elevated RDW levels in patients with COPD were associated with disease severity and lower survival rates. Increased RDW levels might be an indicator of hypoxemia, underlying inflammation and oxidative stress in COPD patients. Therefore, an inexpensive and simple laboratory parameter, such as RDW, could be considered as a biomarker in the evaluation of the severity of COPD.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Author's contributionsKemal Can Tertemiz: Principal study investigator, participated in the design of the study, drafting the manuscript and revising.
Aylin Ozgen Alpaydin: Participated in the design of the study and in drafting the manuscript.
Can Sevinc: Participated in the design of the study, coordination, supervision and important intellectual content.
Hulya Ellidokuz: Participated in the design of the study and performed the statistical analysis.
Ahmet Cagdas Acara: Secondary investigator, studied for data collection and revising the manuscript.
Arif Cimrin: Participated in the design of the study, supervision of the study and important intellectual content.
All authors read and approved the final manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
All coauthors declare that Dr. Kemal Can Tertemiz is responsible for editorial correspondence. This manuscript, including related data, figures and tables has not been published previously and is not under consideration elsewhere.