Sleep disturbance has been described in cystic fibrosis (CF) patients as relevant to clinical and lung function predictive factors helping to improve the diagnosis and early intervention. Related paediatric studies are scarce.
ObjectiveTo describe respiratory sleep disturbance (RSD) and its association with spirometric indices in a population of CF children. A second aim was to determine if spirometric indices and wake-time SpO2 are predictors of sleep disturbance.
MethodsA cross-sectional study involving 33CF paediatric patients. All participants underwent in-lab polysomnography (PSG), pulse oximetry and spirometry. A standardized sleep questionnaire was completed for each patient. Two subgroups were considered: I – Normal (FEV1>−1.64 z-score); II – Obstructed (FEV1≤−1.64 z-score).
ResultsParticipant's median age was 12 (6–18) years, 16 (48.5%) were male. Twenty-nine patients (87.9%) presented sleep complaints. Sleep efficiency was reduced; sleep latency and waking after sleep onset (WASO) increased. N1 increased, N2, N3, REM and awakenings were normal. The apnoea–hypopnoea index was 0.6/h (sd 0.9); respiratory disturbance index (RDI) was 6.6/h (sd 5.2). Mean awaking (97% (sd 1.1)) and sleep SpO2 (95% (sd 2.7)) were normal; mean nocturnal oximetry desaturation index was 2.36/h; minimal nocturnal SpO2 was 89% (sd 4.1).
We found associations between mean nocturnal SPO2 and mean values of FEV1 (r=0.528; p=0.002) and FEF25–75 (r=0.426; p=0.013). There were significant differences in nocturnal SpO2 between normal and obstructed patients (p<0.000). PSG data correlated with the questionnaire answers for night awakenings and WASO (p=0.985) and difficult breathing during sleep and RDI (p=0.722).
This study points to most CF children having sleep complaints, and highlights the correlation between subjective assessment of sleep and PSG and spirometric results. Awake-time SpO2 and spirometric values are possible risk predictors for nocturnal desaturation.
Sleep disturbance in cystic fibrosis (CF) patients has been increasingly recognized in all ages.1–6 Respiratory sleep disturbance (RSD), with nocturnal desaturation4,7 and hypoventilation6 has been described in association with chronic lung disease and respiratory failure1,8 as well as with cough9,10 and upper airway obstruction.11,12 Sleep fragmentation and poor sleep quality are also reported.6,11,13
It is not clear in what way RSD modulates the clinical course of children with CF,14 but it interferes with quality of life.11 Other factors contributing to poor sleep quality in CF patients are night anguish, fear of death, drug effects and the need for nocturnal therapies.11,14,15
Lung function measures, in particular spirometric indices, are critical for the assessment and management of CF patients; they are a primary diagnostic, therapeutic and prognostic endpoint.16–20 Few reports have evaluated lung function tests and awake pulse oxymetry (SpO2) as predictors of sleep disturbance in children with CF and the results are contradictory.6,14,15,21
Our main objective was to describe respiratory sleep disturbance in a population of clinically stable children and adolescents with CF. Our aim was also to identify if spirometric indices (FEV1, FEF25–75, FEV1/FVC) and awake-time SpO2 could be predictors of sleep disturbance.
MethodsThis was an observational, prospective, cross-sectional and descriptive–analytical study. Children were recruited from the Paediatric Unit of a Specialized Centre for Cystic Fibrosis of a Tertiary Care Hospital, between February and October 2013. Written informed consent was obtained from parents of children under 16 years-old and from patients above that age.
All but one patient had CF confirmed by the presence of two transmembrane regulator (CFTR) mutations; one child had one CFTR mutation with a sweat chloride test >60mmol/l, associated with characteristic phenotype; twenty-one (63.6%) were homozygote for the mutation DelF508.
Clinical stability, defined as the absence of respiratory exacerbations and increased tiredness, maintenance of weight gain and stable FEV1, for a month previous to the study was required for inclusion. Exclusion criteria were chronic respiratory failure or being unable to cooperate during spirometry.
Body mass index (BMI) was assessed and reported as z-score.
All participants or their parents completed a standardized sleep questionnaire (SQ) before polysomnography (PSG); daytime SpO2 and spirometry were performed the following day.
SQ is a non validated but routinely applied questionnaire at the Paediatric Sleep Laboratory. SQ has been developed as a rapid screening tool based on the Paediatric Sleep Questionnaire22 and the Sleep Apnea Questionnaire.23 It includes questions about sleep quality, night-time and daytime symptoms and signs of RSD and sleep disturbance, and associated events like parasomnias.
In-lab PSG (SomnoScreen® Plus TM Domino Software, v.2.3.1) was performed for a minimum of 7h. The following parameters were recorded at the same time: six channel electroencephalogram, bilateral electrooculogram, anterior tibialis and chin electromyogram, electrocardiogram, oronasal thermistor airflow detection, nasal cannula transducer, body position, tracheal microphone, thoracic and abdominal movements using respiratory effort bands and pulse oximetry.
A desaturation event was defined as a decrease of SpO2≥3%. Mean and minimum SpO2 were determined as well as the percentage of total sleep time with SpO2<90%. Analysis of sleep stages, arousals, movements and respiratory events was performed according to the American Academy of Sleep Medicine manual.24–26 Flow limitation was defined as a flattening of the inspiratory portion of the flow waveform detected by nasal cannula pressure during sleep without criteria of hipopnoea. The sleep data included were: time in bed, defined as time from lights out to lights on; sleep efficiency (SE), calculated as total sleep time divided by the total time in bed; sleep latency (SL), defined from lights out until the first epoch of any sleep stage; wake after sleep onset (WASO), defined as time spent awake between the sleep onset and the end of sleep; REM latency (REML) defined as time between sleep onset to the first REM epoch. Sleep stages duration is presented as a percentage of total sleep time (TST).
Normative values according to Beck et al.,27 were adopted; respiratory disturbance index (RDI) was defined as the sum of all respiratory events (flow limitations, apnoeas, hipopnoeas) and was interpreted according to Uliel.28
Continuous transcutaneous CO2 (SENTEC® digital V-ST-AT2.02) monitoring was performed to assess median and maximal tcCO2.
Awake SpO2 was assessed by digital measure with Nonin® 7500 (Nonin Medical Inc., Plymouth, MN, USA), the mean of at-rest values obtained over one-minute period of the plestismographic wave form stability, with the patient in a sitting position.
Spirometry (Jaeger Master Screen PFT® – Viasys Healthcare, v.5.3.0) was performed according to the American Thoracic Society and the European Respiratory Society criteria29 for patients ≥6 years of age; values were expressed in z-scores according to the equations from the growing lung initiative (growinglungs.org.uk).30 The sample was then stratified according to FEV1 as Group I, within normal clinical range values (z-score>−1.64) and Group II, classified as obstructive (z-score≤−1.64).
Descriptive statistics, correlations by Pearson's coefficient, Mann–Whitney test and K–Smirnov test were performed as appropriate and a significance level of 5% was considered. Statistical Package for the Social Sciences 21.0 (SPSS, Chicago, IL, USA) was used for all tests.
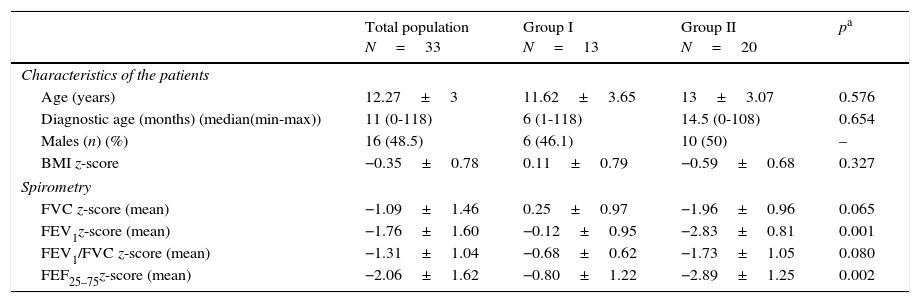
ResultsForty-five CF patients aged between 6 and 18 years old were recruited and 33 were included. Eight patients refused to participate in the study and four were excluded: two had home ventilation or oxygen therapy, one an unconfirmed diagnosis and another was not clinically stable during the study period. Sixteen (48.5%) patients were male; the median age was 12 (6–18) years-old. Median age at CF diagnosis was 11 (0–118) months. Average BMI z-score was normal (−0.35±0.78) (Table 1).
Demographics and spirometry results.
| Total population N=33 | Group I N=13 | Group II N=20 | pa | |
|---|---|---|---|---|
| Characteristics of the patients | ||||
| Age (years) | 12.27±3 | 11.62±3.65 | 13±3.07 | 0.576 |
| Diagnostic age (months) (median(min-max)) | 11 (0-118) | 6 (1-118) | 14.5 (0-108) | 0.654 |
| Males (n) (%) | 16 (48.5) | 6 (46.1) | 10 (50) | – |
| BMI z-score | −0.35±0.78 | 0.11±0.79 | −0.59±0.68 | 0.327 |
| Spirometry | ||||
| FVC z-score (mean) | −1.09±1.46 | 0.25±0.97 | −1.96±0.96 | 0.065 |
| FEV1z-score (mean) | −1.76±1.60 | −0.12±0.95 | −2.83±0.81 | 0.001 |
| FEV1/FVC z-score (mean) | −1.31±1.04 | −0.68±0.62 | −1.73±1.05 | 0.080 |
| FEF25–75z-score (mean) | −2.06±1.62 | −0.80±1.22 | −2.89±1.25 | 0.002 |
FVC – forced vital capacity; FEV1 – forced expiratory volume in 1s; FEF25–75 – forced expiratory flow between 25% and 75% of maximal expiratory flow.
Twenty-nine patients (87.9%) reported poor sleep quality and/or RSD: 19 (57.6%) complained of impaired sleep onset and awaking more than twice per night; 15 (45.5%) reported difficult breathing during sleep and 19 (57.6%) reported snoring, 29 (87.9%) stated they stopped breathing during sleep and 21 (63.7%) were mouth breathers. Daytime symptoms were rare, and the most common was inattentive behaviour (39.4%). More than half of the patients mentioned sleep talking (54.5%). Parasomnias were not relevant.
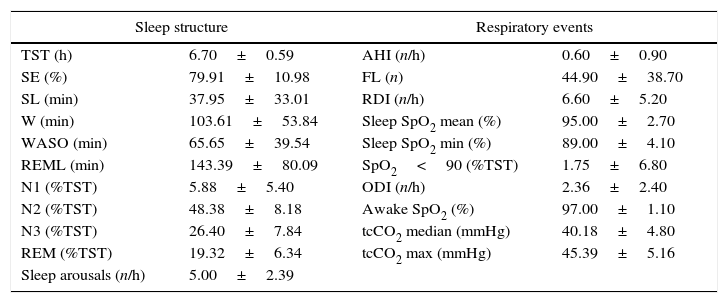
Polysomnography resultsSleep structureMean TST was 6.7±0.59h, with decreased SE (79.91±10.98%) and increased SL (37±33.07min) and WASO (65.65±39.54min). REML was normal (143.39±80.09min), and so was stages distribution N2 (43.8±8.9%TST), N3 (26.4±7.8%TST) and REM (19.32±6.3%TST); N1 was slightly elevated (5.88±5.4%TST) and showed a negative association with sleep efficiency (r=−0.561; p=0.001). The arousal index (5±2.39/h) was within normal range (Table 2).
Polysomnography analysis.
| Sleep structure | Respiratory events | ||
|---|---|---|---|
| TST (h) | 6.70±0.59 | AHI (n/h) | 0.60±0.90 |
| SE (%) | 79.91±10.98 | FL (n) | 44.90±38.70 |
| SL (min) | 37.95±33.01 | RDI (n/h) | 6.60±5.20 |
| W (min) | 103.61±53.84 | Sleep SpO2 mean (%) | 95.00±2.70 |
| WASO (min) | 65.65±39.54 | Sleep SpO2 min (%) | 89.00±4.10 |
| REML (min) | 143.39±80.09 | SpO2<90 (%TST) | 1.75±6.80 |
| N1 (%TST) | 5.88±5.40 | ODI (n/h) | 2.36±2.40 |
| N2 (%TST) | 48.38±8.18 | Awake SpO2 (%) | 97.00±1.10 |
| N3 (%TST) | 26.40±7.84 | tcCO2 median (mmHg) | 40.18±4.80 |
| REM (%TST) | 19.32±6.34 | tcCO2 max (mmHg) | 45.39±5.16 |
| Sleep arousals (n/h) | 5.00±2.39 | ||
TST – total sleep time; SE – sleep efficiency; SL – sleep latency; W – wake; WASO – wake after sleep onset; REML – REM latency; N1 (%TST) – time in N1 (%); N2 (%TST) – time in N2 (%); N3 (%TST) – time in N3 (%); REM (%TST) – time REM (%); AHI – apnoea/hypopnoea index; FL – flow limitation; RDI – respiratory disturbance index; ODI – oxygen dessaturation index; Sleep SpO2 mean – mean sleep pulse oximetry; Sleep SpO2 min – minimal sleep pulse oximetry; tcCO2 – transcutaneous CO2 (median and maximal).
Mean apnoea/hypopnoea index (AHI) was 0.6±0.9/h of sleep; mean flow limitations (44.9±38.7) and RDI (6.6±5.2/h) were slightly increased (Table 2). Twenty-nine (87.9%) patients presented paradoxical breathing and eight (24.2%) snored.
Nocturnal gas exchangeMean awake (97±1.1%) and nocturnal SpO2 (95±2.7%) were in the normal range. Mean oxygen desaturation index (ODI) was 2.36±2.40/h; minSpO2 was 89%±4.1% (Table 2). Average median values of tcCO2 was normal (40.18±4.8mmHg). tcCO2 mean maximal value was 45.39 (±5.16)mmHg.
Awake and sleep SpO2 were positively correlated (r=0.426; p=0.064) and although without statistical significance this may be clinically relevant. The mean ODI showed moderate negative correlation with minimal nocturnal SpO2 (r=−0.532; p=0.001) and with mean nocturnal SpO2 (r=−0.358; p=0.041). A negative moderate correlation was found between awake SpO2 and AHI (r=−0.469; p=0.006).
Spirometric indices analysisMean global z-score was reduced for FEV1 (−1.76±1.60) and for FEF25–75 (−2.06±1.62). Thirteen patients had FEV1 within the normal range (Group I) and 20 patients had FEV1≤−1.64 z-score and were classified as obstructed (Group II). Mean z-score for FVC and for FEV1/FVC was normal (Table 1).
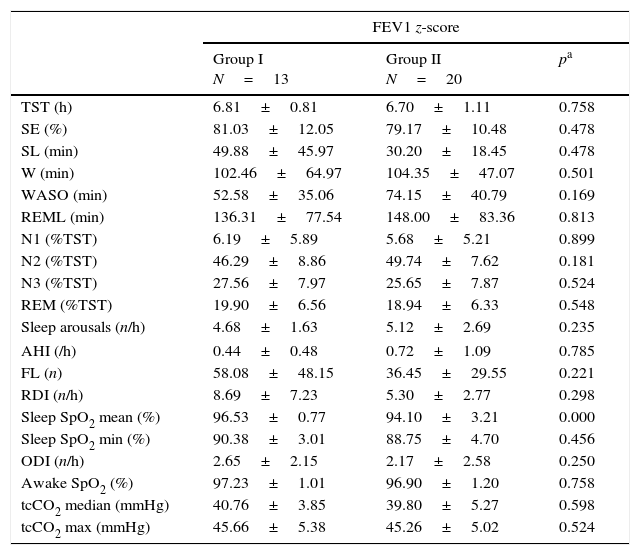
Polysomnographyc and spirometric results association analysisAs defined by FEV1, compared to Group I, patients from Group II showed a trend towards a lower mean SE (81.03±12.05% vs 79.17±10.98%; p=0.478), more sleep arousals (4.69±1.63/h vs 5.12±2.69/h; p=0.235) and increased WASO (52.58±35.06min vs 74.12±40.79min; p=0.169), although none reached statistical significance (Table 3).
Polysomnography events according to FEV1z-score group analysis.
| FEV1 z-score | |||
|---|---|---|---|
| Group I N=13 | Group II N=20 | pa | |
| TST (h) | 6.81±0.81 | 6.70±1.11 | 0.758 |
| SE (%) | 81.03±12.05 | 79.17±10.48 | 0.478 |
| SL (min) | 49.88±45.97 | 30.20±18.45 | 0.478 |
| W (min) | 102.46±64.97 | 104.35±47.07 | 0.501 |
| WASO (min) | 52.58±35.06 | 74.15±40.79 | 0.169 |
| REML (min) | 136.31±77.54 | 148.00±83.36 | 0.813 |
| N1 (%TST) | 6.19±5.89 | 5.68±5.21 | 0.899 |
| N2 (%TST) | 46.29±8.86 | 49.74±7.62 | 0.181 |
| N3 (%TST) | 27.56±7.97 | 25.65±7.87 | 0.524 |
| REM (%TST) | 19.90±6.56 | 18.94±6.33 | 0.548 |
| Sleep arousals (n/h) | 4.68±1.63 | 5.12±2.69 | 0.235 |
| AHI (/h) | 0.44±0.48 | 0.72±1.09 | 0.785 |
| FL (n) | 58.08±48.15 | 36.45±29.55 | 0.221 |
| RDI (n/h) | 8.69±7.23 | 5.30±2.77 | 0.298 |
| Sleep SpO2 mean (%) | 96.53±0.77 | 94.10±3.21 | 0.000 |
| Sleep SpO2 min (%) | 90.38±3.01 | 88.75±4.70 | 0.456 |
| ODI (n/h) | 2.65±2.15 | 2.17±2.58 | 0.250 |
| Awake SpO2 (%) | 97.23±1.01 | 96.90±1.20 | 0.758 |
| tcCO2 median (mmHg) | 40.76±3.85 | 39.80±5.27 | 0.598 |
| tcCO2 max (mmHg) | 45.66±5.38 | 45.26±5.02 | 0.524 |
Mann–Whitney test.
TST – total sleep time; SE – sleep efficiency; SL – sleep latency; W – wake; WASO – wake after sleep onset; REML – REM latency; N1 (%TST) – time in N1 (%); N2 (%TST) – time in N2 (%); N3 (%TST) – time in N3 (%); REM (%TST) – time REM (%); AHI – apnoea/hypopnoea index; FL – flow limitation; RDI – respiratory disturbance index; ODI – oxygen dessaturation index; sleep SpO2 mean – mean sleep pulse oximetry; sleep SpO2 min – minimal sleep pulse oximetry; tcCO2 – transcutaneous CO2 (median and maximal).
No differences between groups were found for respiratory events during sleep. A significant difference was found for mean sleep SpO2, lower in Group II (94.10±3.21% vs 96.53±0.77%; p<0.000); minimal sleep SpO2 (88.75±4.77% vs 90.38±3.01) was also without statistical significance (p=0.456). Mean median tcCO2 was normal in both groups (Table 3). Analysis for FEF25–75 presented similar results.
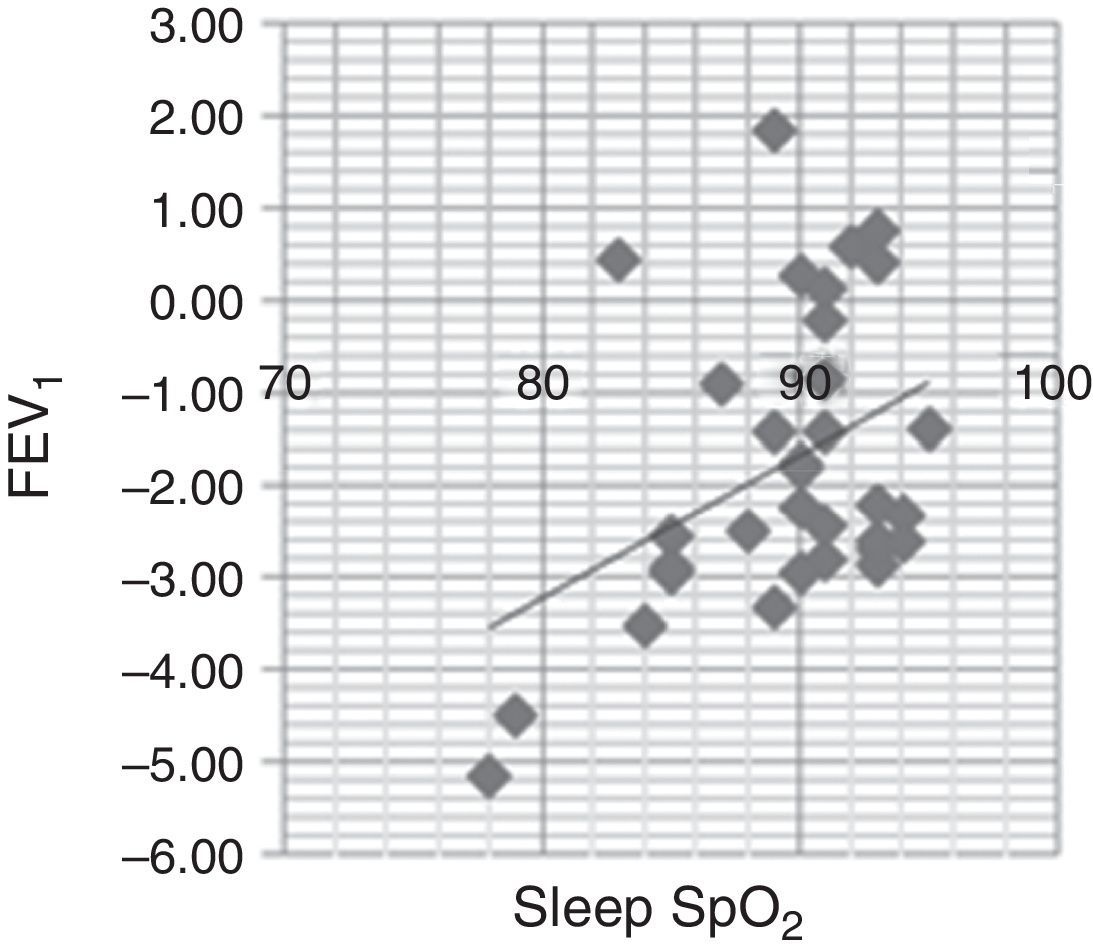
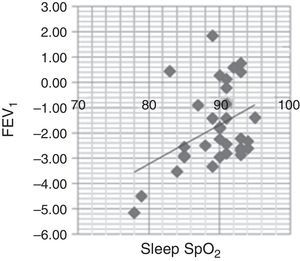
FEV1 was positively associated with average sleep SpO2 (r=0.528; p=0.002) (Fig. 1) and minimal sleep SpO2 (r=0.405; p=0.019). A moderate, negative association between awake SpO2 and AHI (r=−0.469; p=0.006) was found.
Maximal tcCO2 was negatively and moderately associated with average sleep SpO2 (r=−0.387; p=0.026).
Polysomnography data and sleep questionnaire Association analysisPSG data significantly correlated with the questionnaire answers for night awakenings and WASO (p=0.985), and for difficult breathing during sleep and RDI (p=0.722). Difficulty in falling asleep correlated moderately with SL (p=0.345). No association was found for snoring reported in the SQ and in the PSG (p=0.039) or for breathing interruption during sleep (p=0.039) and AHI (p=0.049).
DiscussionTo the best of our knowledge this is the first study in a CF Portuguese Paediatric population to evaluate sleep disturbance and to try to relate it to spirometric indices, a clinical diagnostic and prognostic relevant endpoint.16
There are some studies involving adult CF patients and CF children but only during acute illness or with higher clinical severity.1,7,13,31 Most studies presented limitations such as small population samples, sleep assessment performed only through sleep questionnaires and/or evaluated by pulse oximetry analysis.6,15,32 Only a few studies assessed sleep with PSG or quantified severity of lung disease with an objective measure like spirometry, as we did.1,7,11,13,33
In this study, 87.9% of clinically stable children between six and eighteen years, with normal or mildly affected lung function, were identified as having sleep disturbance, either respiratory or of the sleep structure. These alterations correspond to subjectively diminished quality of sleep, even with slight anomalies of PSG values. These findings are consistent with the majority of other reports showing that CF patients have lower sleep efficiency and quality.6,11,13,15,34
Difficulty in breathing during sleep was reported in almost half of our patients and had a positive association with RDI. Night awakenings and WASO (p=0.985) were also associated. These results confirm what was suggested by Milross,33 that even a simple questionnaire like the one used in this study has an important role in sleep disturbance screening in CF patients.
The in-lab first night effect must be considered when evaluating SE and SL disturbance: sleep in the laboratory can influence sleep onset and duration, as showed by Verhulst et al.35 who described the first night effect in children with RSD. This study included predominantly adolescents, and sleep phase delay associated with this age group may act as a confounder to the sleep onset and duration compromise.36 As in our study, Perin and Milross also found higher wakefulness and sleep latency leading to lower sleep efficiency in CF patients.13,34
In the present study, arousal index was normal but higher in the group with higher respiratory compromise evaluated by spirometic indices, as described by Naqvi and Dancey11,31. N1 was elevated and showed a negative association with sleep efficiency, pointing to sleep fragmentation. The higher WASO was more evident in the group with abnormal FEV1, but no significant correlation was found.
Naqvi et al.11 described an association between the magnitude of sleep structure disruption and the severity of lung disease, but not with hypoxaemia or hypoventilation during sleep, which is similar to our results. We could not stratify patients by severity levels because of the small size of the sample, but sleep structure compromise showed a tendency to be more common in the group with lower FEV1.
Ramos et al. studied a stable paediatric CF population and described important complaints about sleep quality and significant architecture disruption as well as prevalent respiratory sleep disturbance and episodes of nocturnal desaturation.14,15 No significant respiratory events were registered in our study. The absence of major respiratory disturbance can be explained by the young age of the patients, the clinical stability and lung function preservation in this sample. Perin did not find higher prevalence of respiratory sleep disturbance either, in an adult CF population with similar characteristics.34 We found a lower SpO2 during sleep, as expected, and although not in the pathological range, there were some sleep desaturations, which are in accordance with other studies: Ramos et al., in a group of school age CF children and adolescents identified an intermittent fall of nocturnal SpO2 and normal or minor lung function compromise.14 Naqvi has also found reduced minimal SpO2, without significant respiratory events. This data is consistent with our findings, as we identified diminished minimal SpO2 associated with ODI, but without significant respiratory events. This author states that apnoea or hypopnoea is uncommon in CF, even in the presence of moderate to severe lung disease.11
Like Milross,13 we found an association between awake and sleep SpO2, that even without statistical significance, can point towards wake oximetry as a possible predictive tool for nocturnal hypoxaemia. However, our sample is small and inter-patients variability of nocturnal SpO2 was high, which precludes us from clearly stating this.
Another interesting finding was the association of awake SpO2 with AHI, which favours the importance of wake oximetry to anticipate the occurrence of nocturnal respiratory events. These findings must be confirmed in larger samples and more heterogeneous populations.
The mild alterations of lung function can be explained by the clinical stability of this paediatric patient sample, due to progress made in CF patient care that postpones more severe disease stages for adult age.34 Our analysis was done with spirometric indices (FEV1, FEF25–75 and FEV1/FVC) above or under the cut-off for normal stated clinical z-scores and we did not include in this study the analysis of other clinical or radiological severity scores, which may limit the extrapolation of results. However, spirometry by itself is accepted as a marker of disease severity,18–20,28,37 conferring reliability to our findings.
We identified significant lower sleep SpO2 in patients with FEV1z-score under normal range, which indicates spirometric indices as possible predictors of nocturnal desaturation, as described in other studies.4,7,15,38,39
As in other studies, we did not find any association between sleep architecture parameters and spirometry.4,7,39
This study has a few limitations: the in-lab night study may not reflect the usual sleep behaviour; patient stability may underestimate the relationship between sleep disturbance and disease severity; the small sample dimension precluded us from doing severity stratification, which could have been more accurate in identifying sleep and wake respiratory compromise; we did not used a quality of life questionnaire, which could have provided important data on sleep disturbance consequences.
Nevertheless this study forms the basis for a relevant discussion about sleep compromise in a paediatric “healthy” group of CF patients. As sleep quality is related to quality of life and maintenance of good clinical condition, the results of this study indicate that sleep evaluation must be considered in the routine assessment of CF patients from early stages of disease.
ConclusionThis study showed that most CF children have complaints about sleep quality, identified by a SQ and confirmed by PSG.
No clear association between sleep structure disruption and the severity of lung disease was found, but although not remarkable, nocturnal O2 desaturation occurred in a clinically stable sample of CF paediatric patients associated with respiratory disease severity assessed by spirometry.
Further studies are needed in more representative populations with a higher clinical severity span to confirm our results.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study would not be possible without children and parents participation, to all thank you.
We also acknowledge Teresa Fonseca, PhD, whose contribution was relevant for the analysis performed.