Obstructive sleep apnea has been associated with higher cancer incidence and mortality. Increased melanoma aggressivity was reported in obstructive sleep apnea patients. Mice exposed to intermittent hypoxia (IH) mimicking sleep apnea show enhanced melanoma growth. Markers of melanoma progression have not been investigated in this model.
ObjectiveThe present study examined whether IH affects markers of melanoma tumor progression.
MethodsMice were exposed to isocapnic IH to a nadir of 8% oxygen fraction for 14 days. One million B16F10 melanoma cells were injected subcutaneously. Immunohistochemistry staining for Ki-67, PCNA, S100-beta, HMB-45, Melan-A, TGF-beta, Caspase-1, and HIF-1alpha were quantified using Photoshop.
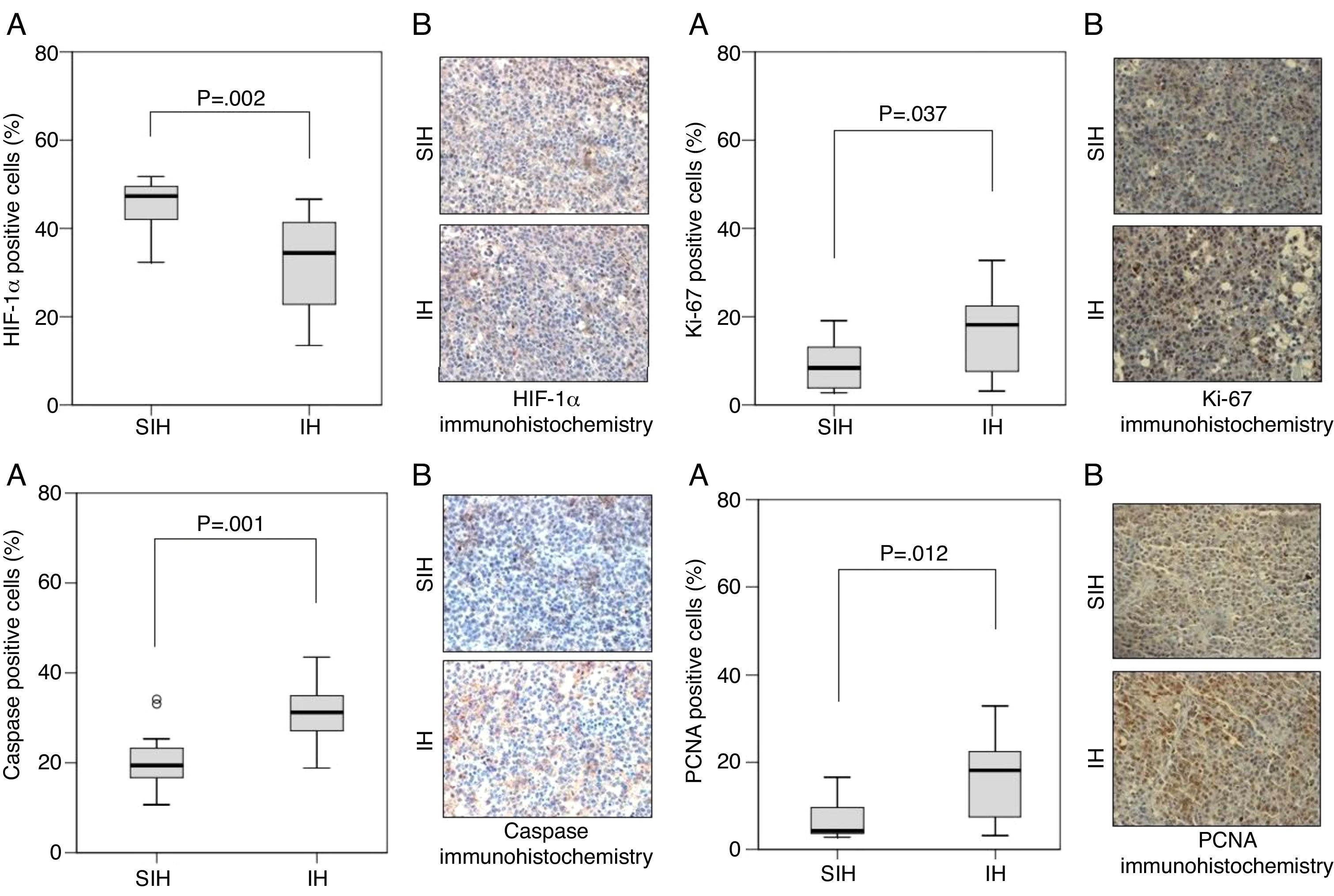
ResultsPercentage of positive area stained was higher in IH than sham IH group for Caspase-1, Ki-67, PCNA, and Melan-A. The greater expression of several markers of tumor aggressiveness, including markers of ribosomal RNA transcription (Ki-67) and of DNA synthesis (PCNA), in mice exposed to isocapnic IH than in controls provide molecular evidence for a apnea–cancer relationship.
ConclusionsThese findings have potential repercussions in the understanding of differences in clinical course of tumors in obstructive sleep apnea patients. Further investigation is necessary to confirm mechanisms of these descriptive results.
Epidemiological data connects obstructive sleep apnea (OSA) with cancer mortality and incidence1–4 as well as with melanoma aggressivity.5 Intermittent hypoxia (IH), a feature of OSA,6 is used as a model to investigate outcomes of this disease.7 Exposure to 6h of one episode of hypoxia per minute to an oxygen nadir of 5% augments melanoma growth8 and metastasis in mice.9 In this model, both obesity and IH affect melanoma growth, with non-addictive effect.10,11
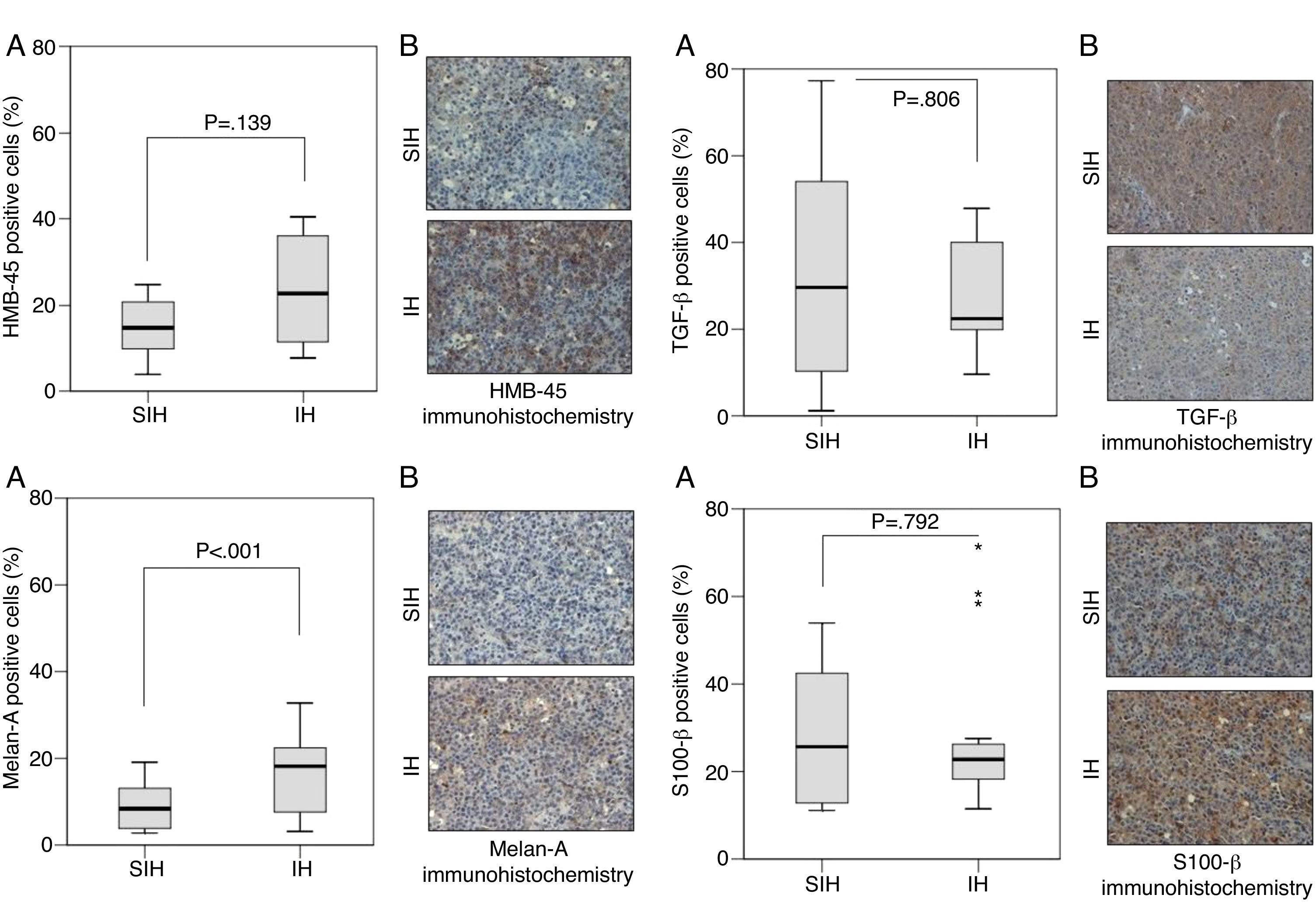
Several proteins are altered specifically in melanoma and have prognostic value. S100 calcium binding protein B (S100-β) is a prognostic marker for melanoma stage, disease recurrence, and low overall patient survival12 Augmented expression of proliferation marker Ki-67 indicates increased ribosomal RNA transcription.13 Greater expression of proliferating cell nuclear antigen (PCNA) in the cell nucleus indicates greater DNA synthesis. The presence of hypoxia-inducible factor 1-alpha (HIF-1α) suggests the exposure of the cell to hypoxia. The protein transforming growth factor beta (TGF-β) controls proliferation, differentiation, and other functions in cells. Caspase-1 induces cell apoptosis and is a marker of malignancy. Melan-A and human melanoma black 45 (HMB-45) are antigens markers of melanocytes, used to identify a tumor as a melanoma.14
The various stages of tumor formation and progression can be regulated by hypoxia.15–18 Knowledge, however, focusing specifically on IH simulating OSA and its molecular effects in melanoma aggressiveness is incipient.
The aim of the present study is to test the hypothesis that an animal model simulating OSA intensifies the expression of aggressiveness markers. In this investigation, mice were exposed to 14 days of isocapnic IH, similar to severe OSA.
MethodsAnimalsTwelve 2-month-old male C57BL/6 mice, weighing between 25 and 30g at the beginning of the study, were used in the study; six exposed to isocapnic IH and six controls exposed to sham IH. Except for the gas mixture, both groups of melanoma-injected mice were subjected to the same protocol. The animals were housed under temperatures ranging between 22.5 and 24.5°C with a 12:12-h light-dark cycle and received ad libitum standard mice chow and water. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Hospital de Clínicas de Porto Alegre (Permit Number: 09-483) and conducted in accordance with the Guide for the Care and Use of Laboratory Animals.19
Isocapnic intermittent hypoxiaThe isocapnic IH system was described in detail before.20–22 In brief, for 14 days, 8h per day, from 9AM to 5PM, in the lights on period, the animals were kept in the hypoxia system. During 30s a mixture with 92% nitrogen and 8% CO2 was released into the hypoxia chamber. A nadir of inspired oxygen fraction of 7±1% and a peak of inspired carbon dioxide of 6±1% were reached as confirmed by sensors inside the cage. Next, room air was insufflated during 30s to restore normoxia. The exposure to isocapnic IH started on the same day as the injection of melanoma cells.
Melanoma cellsTo induce melanoma in the mice, 1×106 B16F10 cells (ATCC-CRL-6475; American Type Culture Collection, Manassas, VA, USA) were used. The cells were suspended in 150μl of phosphate buffered saline and injected subcutaneously in the left hind limb of each mouse.
On the last day of the experiment, each mouse was removed from the IH system to be immediately anesthetized with ketamine (100mg/kg) and xylazine (10mg/kg) intraperitoneally. The animals were not weighed at this moment. After deep anesthesia was confirmed, the tumors were excised and fixed. After tumor excision the mice were euthanized in a CO2 chamber and the organs were macroscopically examined for metastasis.
Hematoxylin-eosin and immunohistochemistryThe paraffin blocks of the tumor were sectioned in 5μm slices. Sections were mounted on glass slides, stained with hematoxylin-eosin, and the tumor diameters measured. The immunohistochemical staining was processed using primary monoclonal mouse antibodies against: (1) HIF-1α (1:200 dilution; sc-53546), (2) TGF-β (1:200 dilution; sc-398), (3) S100-β (1:400 dilution; sc-28533), (4) Melan-A (1:200 dilution; sc-20032), (5) Caspase-1 (1:200 dilution; sc-56036) (all Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), (6) HMB-45 (1:100 dilution; HMB-45; Dako, Carpinteria, CA, USA), (7) Ki-67 (1:50 dilution; SP6; Cell Marque, Rocklin CA, USA), and (8) PCNA (1:200 dilution; 307901; BioLegend, San Diego, CA, USA).
The secondary antibody was PicTure™-MAX Polymer Detection kit, 87-8983 (Invitrogen, Camarillo, CA, USA), which reacts with mouse antibodies. The color was developed with a DAB Chromogen. Sections were counterstained with hematoxylin-Harris and rinsed with ammoniacal water to obtain a light blue color.
Three pictures of each slide at 400× were quantified for immunohistochemistry staining by Adobe Photoshop™. Two different readers, blind to the exposure groups performed the staining analyses. The readings were averaged. When two measurements disagreed by more than 5%, readings from a third observer were utilized instead of the one with greater disparity among the three.23
Statistical analysisStatistical analysis was performed using SPSS (SPSS, Chicago, IL). Medians and quartiles [25th–75th] were utilized to express grouped data. Mann–Whitney test was used to compare measurements of percentage of stained area. Results were considered significant when the probability of alpha error was <0.05.
ResultsThe median tumor diameter in the hypoxia group, was 9mm [interquartile range (IQR): 6–11mm], 50% larger than in the control group, 6mm [IQR: 4–13mm]. The difference, however, did not reach significance (P=0.6). No macroscopic evidence of metastases was observed in brain and lungs of mice.
Caspase-1, Ki-67, and PCNA were significantly different between hypoxia and control groups in terms of the percentage of positive area of the markers (Fig. 1). HIF-1α and Melan-A had significant differences between hypoxia and control groups only in percentage of positive area; HMB-45, and TGF-β had not significant differences between hypoxia and control groups; S100-β had similar percentage of positive area in the hypoxia and control groups (Fig. 2).
Our results indicate that an isocapnic IH model, mimicking OSA, increases the aggressiveness of melanoma. The tumors from the hypoxia group can be considered of higher grade than the ones from the control group. This is the first report of increased expression of Ki-67 and PCNA by IH. Besides supporting previous findings,7–10 the present results suggest a malignancy-enhancing effect of OSA.
The PCNA is a protein essential both for DNA replication and repair used as a proliferation marker. It participates as a DNA polymerase accessory in the synthesis of DNA during the S phase and interacts with cellular proteins involved in the regulation of cell cycle.24,25 Enhanced expression of PCNA is associated with increases in metastasis and with lower survival rates. In advanced gastric cancer,26 proliferation indices, i.e., the percentage of cells dividing, above 50% as marked by either PCNA or Ki-67 are associated with a three times higher mortality at 40 months.
Ki-67, a nuclear and nucleolar protein linked to the proliferation of somatic cells, is expressed in phases G1, S, G2, and mitosis, but not in the G0 phase. This makes Ki-67 one of the most often utilized markers to determine the fraction of proliferating cells in various tumors. The expression of Ki-67 in melanocytic lesions has a role as diagnostic biomarker, and a marker of poor prognosis.27 Anorectal melanoma patients with 40% or more of the nuclei positive for Ki-67 are all deceased in 40 months, while less than 40% of the patients with Ki-67 scores below this threshold died in the same period. No patient with more than 80% of PCNA positive nuclei survived more than 30 months while about 60% of the patients with lower PCNA scores were alive at this time point.28
In the present study, the tumor size of the hypoxia group, despite being 50% larger than that of the control group, is non-significantly different. Previous reports7–10 utilized longer duration of exposure and IH with a lower inspired oxygen nadir than the present experiment. Since the tumor growth is exponential, it might have been possible to reach a significant difference in size by allowing a few more days of exposure. Although all animals were injected with the same number of cells, each tumor may derive from implants of different sizes, increasing the variability of this measurement. Additionally, unexpected cell lysis during injection could have triggered inflammatory/immune responses that would alter tumor growth, increasing the unevenness of tumor size.
No metastases were observed in our study after 14 days, agreeing with the literature on this subject. Under IH, Almendros et al.9 have observed spontaneous lung metastases after 30 days of the tumor cells injection. In their study, 13 out of 18 mice under IH survived until day 30. The veterinarian in charge of our animals considered that prolonging the study beyond 14 days would cause unnecessary suffering to the mice. The model used by them, with tumor cells being injected in a tail vein proved to be a suitable way to obtain metastasis in 21 days, before tumor growth causes death and distress to the experimental animals.
Melanoma showed to be an appropriate cancer model to test the hypothesis of hypoxia affecting cancer aggressiveness. More than 430 hypoxia-responsive genes have been identified in the B16F10 murine melanoma cell line, in vitro.29
The finding of HIF-1α expression being lower under IH is an unexpected finding. In presence of hypoxia, ubiquitination of HIF-1α is reduced, allowing the protein to accumulate.30 IH, however, occurring during 8h per day, is a much more complex phenomenon than constant hypoxia. In apnea-like IH the tissues undergo hypoxic episodes of short duration. In our model, hypoxia totals 4h, i.e., 480 cycles with 30s of declining PIO2. The rest of the day, for around 16h per day out of the hypoxic chamber plus 4h of room air breathing at each 30-s recovery period, the cells are in their usual normoxic conditions. This permits full recovery of HIF-1α levels from the regulating processes that would increase its expression. A model of IH alternating 1h of 1% oxygen with 30min of 20% oxygen promotes angioneogenesis.16 It is possible to speculate that the IH promoted formation of extra blood vessels, enhancing oxygen supply to tumor cells.
Greater positive area of Melan-A staining in the IH group may indicate a greater expression of melanocytic proteins.31 This is indicative of melanocytic proliferation but not necessarily of greater malignancy.
One limitation of the present study is the lack of power to accept the negative results. At the time the experiments were carried out no evidence of a cancer-IH relation existed. The ethics committee insisted on a small number of animals in this first exploratory phase. The positive findings, however, based in immunohistochemistry are solid enough to originate future research. An ideal project should include genetic and pharmacological interventions that would allow understanding of the mechanisms activated by the IH. The investigation of mechanisms, however, is outside the scope of the search for melanoma markers that might be affected in OSA.
The enhanced expression of markers of tumor aggressiveness under IH may suggest a molecular basis for the apnea-cancer relationship. Additional investigation of the molecular mechanisms triggered by IH that are relevant to malignancy is warranted by these results.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
This study was supported by the Research Incentive Fund of the Hospital de Clínicas de Porto Alegre (FIPE – HCPA), Porto Alegre, Brazil. We are indebted to the pathologists Drs. Dennis Baroni and Maria Isabel Edelweiss assistance in the tumor assessment.