Tuberculosis (TB) incidence declined in Portugal in recent decades, but trends differ between regions and population subgroups. We investigated these differences to inform prevention and control programmes.
MethodsWe extracted TB notifications from the Portuguese National TB Surveillance System (SVIG-TB) in 2010–2017, disaggregated by region, age group, nationality and HIV status. We calculated notification rates using denominators from the Portuguese National Institute of Statistics and the Joint United Nations Programme on HIV/AIDS and performed stratified time series analysis. We estimated interannual decline percentages and 95% confidence intervals (CI) using Poisson and binomial negative regression models.
ResultsThe overall TB notification rate decreased from 25.7 to 17.5/100,000 population from 2010 to 2017 (5.2%/year) in Portugal. Interannual decline did not differ significantly between regions, but it was smaller amongst non-Portuguese nationals (-1.57% [CI: -4.79%, 1.75%] vs -5.85% [CI: -6.98%, -4.70%] in Portuguese nationals); children under five years of age (+1.77% [CI: -4.61%, 8.58%] vs -5.38% [CI: -6.33%, -4.42%] in other age groups); and HIV-negative people (-6.47% [CI: -9.10%, -3.77%] vs -11.29% [CI; -17.51%, -4.60%] in HIV-positive).
ConclusionsThe decline in TB notification rates in Portugal during the study period has been steady. However, the decline amongst non-Portuguese nationals, children under five years of age and non-infected-HIV patients was lower. No significant differences were observed between regions. Changes in TB epidemiology in specific risk groups and geographical areas should be closely monitored to achieve the objectives of the End TB Strategy. We recommend intensifying screening of TB in the subpopulations identified.
Tuberculosis (TB) incidence is steadily declining in Europe, although it remains high worldwide and in some European countries. TB is still as a major public health problem, also due to drug-resistant forms and with HIV-coinfection.1,2 In the World Health Organization (WHO) European Region and in the European Union/European Economic Area (EU/EEA) the average annual decline of the TB incidence rate was 5.1% between 2015 and 2019 (11.9 to 9.6/100,000 inhabitants from 2015 to 2019 in EU/EEA).1 In Portugal, which has one of the highest notification rates in the EU/EEA, the average annual decline was 5.0% (from 21.2 to 17.2/100,000).1 Out of all cases notified in 2019 in the EU/EEA, 76.9% were new cases and 9.7% had been previously treated for TB (13.4% with unknown previous history of TB).1
The WHO End TB strategy states that to achieve a reduction of 80% in new TB cases and 90% in deaths by 2030, collaborative TB/HIV activities, and management of co-morbidities are needed.2
As for other infectious diseases, we can describe TB as syndemic: it is an old endemic disease, presenting a synergic interaction with and worsening the independent damages of other diseases or conditions such as HIV, diabetes, or many social determinants of health (deprivation and inequities of health amongst others).3,4 TB affects differently specific population groups and geographical areas. amongst others, risk groups include specific clinical conditions (such as HIV or other immunosuppression), close contact with a TB case or migration from an area with a high incidence of TB, as well as unfavoured socioeconomic and occupational groups.5 In some risk groups, like HIV-infected, a decline in TB incidence rates was observed in the EU/EEA and in Portugal between 2015 and 2019. The relative weight of other groups, such as children under five years of age and immigrants, increased despite the overall decline in incidence.1 In Portugal regional differences in risk of TB have been reported, with more densely populated regions such as Lisbon and Tagus Valley (LTV) and North regions being more affected.6-8
To analyse the differences in magnitude of decline and to better understand risk from surveillance data, further analysis of the available data is needed. We aimed at identifying population groups and geographical areas in Portugal with smaller decline in TB notification rates, to inform targeted prevention and control interventions.
MethodsStudy design and participantsTime trend analysis study. We performed a time series analysis of active TB cases notified to the Portuguese TB clinical notification and follow-up surveillance system (SVIG-TB), including cases diagnosed between 2010 and 2017.
Case definition and TB surveillance system (SVIG-TB)All confirmed, probable, and possible cases of TB diagnosed in 2010–2017 were retrieved from SVIG-TB. The case definition used is in line with EU case definitions.9 Cases are notified to SVIG-TB by any physician diagnosing and following-up TB patients, mostly at TB outpatient centres (part of public primary care). The notifications are based on paper forms that are subsequently computerised and centralised at the regional and national levels, and include clinical, epidemiological and laboratory data, from diagnosis to discharge.10
Definition of variables and potentially associated factorsSociodemographic and medical characteristics of TB patients were collected from SVIG-TB. We analysed the variables Sex (Female, Male), Age (<5 and 5 or more), Nationality (Portuguese, non-nationals), Site of infection (Extrapulmonary, Pulmonary), HIV status (Positive, Negative), and region of residence (North, Centre, Lisbon and Tagus Valley [LTV], Alentejo, Algarve, Azores, Madeira).
DenominatorsWe obtained denominators for each analysed year by Region, Age, Sex and Nationality from the Portuguese National Institute for Statistics11 and estimates of people living with HIV in Portugal from Joint United Nations Programme on HIV/AIDS (UNAIDS) data 2019 report.12
Statistical analysesPatients were described using counts and proportions for each of the categorical independent variables included. Age was described as Median (IQR) and proportion of <5 years old. Monthly and yearly notification rates of TB were calculated as number of cases notified per 100,000 inhabitants. This was done overall and stratified by sex, age group, nationality, and region of residence. Further indicators of yearly notification rates of HIV+ and HIV- TB, Pulmonary and Extrapulmonary TB were computed overall. Interannual declines with 95% confidence intervals (CI) for each group were estimated using Poisson regression models. If overdispersion was observed the interannual decline was estimated using negative binomial regression. Analyses were performed using STATA (version 14; Stata Corporation, College Station, TX, USA).
Ethical approvalIn this study, patients were not directly involved; anonymized data were extracted from the national TB clinical notification and follow-up surveillance system (SVIG-TB) and analysed by authors at Directorate-General of Health, as part of their routine functions of surveillance and control of communicable diseases. The study was performed following the indications of the Helsinki Declaration (reviewed in Tokyo, October 2004).
Informed consentPatients’ data were anonymized prior to analysis and, for that reason, informed consent was not required.
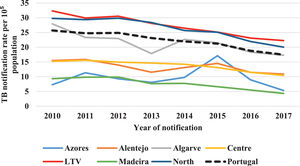
ResultsFrom 2010 to 2017, the yearly notifications of TB in Portugal decreased from 2715 to 1800, corresponding to 25.7 and 17.5 notifications per 100,000 population, respectively (global decrease of 33.7% and a yearly decrease of 5.2%). The regions with greatest reduction in incidence were Madeira (56.0%), Algarve (39.7%) and Alentejo (35.3%) (Fig. 1, Supplementary Table S1). Madeira had the greatest yearly decrease in incidence, followed by Algarve (5.4%), North (5.4%) and Lisbon and Tagus Valley (LTV) (5.2%) (Table 1).
Tuberculosis notification rate decline (interannual change) per 100,000 population and comparative average difference rate by region in Portugal, 2010–17.
Amongst the 18,550 TB cases included in the analysis, 12,504 were confirmed (67.4%), 3122 probable (16.8%) and 2924 possible (15.8%). Three out of the seven Portuguese regions represented 91.6% of all the notifications (LTV 41.6%, North 36.4%, and Centre 13.6%). Proportion of male cases was 65.6% overall (highest in Algarve, 68.4%, and lowest in LTV, 63.0%). The median age was 47 years old (lowest in LTV, 44, highest in Centre region, 49); patients under 5 years of age represented 1% of all cases (highest proportions in Madeira and North region – 1.3% and 1.2%, respectively – and lowest in Centre region, 0.6%). The proportion of Portugal-born cases was 85.8% (lowest LTV, 73.3%, and highest North region and Madeira – 96.5% and 95.6%, respectively).
Regarding clinical characteristics, 10.7% of all notified cases were HIV-infected patients. HIV coinfection prevalence was highest in LTV and Algarve (16.2% and 11.8%, respectively) and lowest in Azores and Madeira (1.6% and 3.8%, respectively). Extrapulmonary TB accounted for 27.9% of all cases (proportion ranging from 9% in Azores to 30.7% in LTV) (Table 2).
Sociodemographic and medical characteristics of tuberculosis patients notified by region in Portugal, 2010–2017 (N = 18,550).
| Portugal | Region | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Centre | LTV* | North | Alentejo | Algarve | Azores | Madeira | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Total | 18,550 | 2525 | 13.6 | 7,7 | 41.6 | 6745 | 36.4 | 452 | 2.4 | 763 | 4.1 | 190 | 1.0 | 159 | 0.9 | |
| Sex | ||||||||||||||||
| Female | 6380 | 34.4 | 822 | 32.6 | 2857 | 37 | 2222 | 32.9 | 128 | 28.3 | 241 | 31.6 | 56 | 29.5 | 54 | 34 |
| Male | 12,170 | 65.6 | 1703 | 67.4 | 4859 | 63 | 4523 | 67.1 | 324 | 71.7 | 522 | 68.4 | 134 | 70.5 | 105 | 66 |
| Age | ||||||||||||||||
| Median (IQR) | 47 (34, 61) | 49 (36, 65) | 44 (33, 59) | 48 (36, 62) | 53 (39, 71) | 47 (35, 64) | 46.5 (34, 56) | 46 (36, 55) | ||||||||
| Under 5 | 174 | 1.0 | 15 | 0.6 | 67 | 0.8 | 81 | 1.2 | 4 | 0.9 | 5 | 0.7 | 0 | 0 | 2 | 1.3 |
| >5 years | 18,350 | 98.9 | 2510 | 99.4 | 7629 | 98.9 | 6664 | 98.8 | 448 | 99.1 | 758 | 99.3 | 184 | 96.8 | 157 | 98.7 |
| Missing | 26 | 0.1 | 0 | 0 | 20 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 3.2 | 0 | 0 |
| Nationality | ||||||||||||||||
| Portuguese | 15,909 | 85.8 | 2405 | 95.3 | 5654 | 73.3 | 6513 | 96.5 | 420 | 93.0 | 612 | 80.2 | 153 | 80.5 | 152 | 95.6 |
| Non-nationals | 2421 | 13.1 | 117 | 4.6 | 1887 | 24.4 | 229 | 3.4 | 30 | 6.6 | 151 | 19.8 | 0 | 0 | 7 | 4.4 |
| Missing | 220 | 1.1 | 3 | 0.1 | 175 | 2.3 | 3 | 0.1 | 2 | 0.4 | 0 | 0 | 37 | 19.5 | 0 | 0 |
| HIV status | ||||||||||||||||
| Positive | 1980 | 10.7 | 151 | 6.0 | 1248 | 16.2 | 460 | 6.8 | 22 | 4.9 | 90 | 11.8 | 3 | 1.6 | 6 | 3.8 |
| Negative | 13,801 | 74.4 | 2040 | 80.8 | 5209 | 67.5 | 5353 | 79.4 | 373 | 82.5 | 635 | 83.2 | 69 | 36.3 | 122 | 76.7 |
| Missing | 2769 | 14.9 | 334 | 13.2 | 1259 | 16.3 | 932 | 13.8 | 57 | 12.6 | 38 | 5.0 | 118 | 62.1 | 31 | 19.5 |
| Site of infection | ||||||||||||||||
| Extrapulmonary | 5186 | 27.9 | 668 | 26.5 | 2371 | 30.7 | 1796 | 26.6 | 101 | 22.3 | 185 | 24.2 | 17 | 9.0 | 48 | 30.2 |
| Pulmonary | 13,261 | 71.5 | 1833 | 72.6 | 5275 | 68.4 | 4944 | 73.3 | 351 | 77.7 | 575 | 75.4 | 172 | 90.5 | 111 | 69.8 |
| Missing | 103 | 0.6 | 24 | 0.9 | 70 | 0.9 | 5 | 0.1 | 0 | 0 | 3 | 0.4 | 1 | 0.5 | 0 | 0 |
The interannual decline in notification rate was significantly smaller in non-Portuguese nationals (−1.57%, CI: −4.79%, 1.75%) than in Portuguese-born individuals (−5.85%, CI: −6.98%, −4.70%); in children under five years (+1.77%, CI: −4.61%, 8.58%) than in other ages (−5.38%, CI: −6.33%, −4.42%); and in HIV-negative people (−6.47%, CI: −9.10%, −3.77%) than in HIV-positive (−11.29%, CI; −17.51%, −4.60%) (Table 3).
Tuberculosis notification rate decline (interannual change) per 100,000 inhabitants by population group in Portugal, 2010–17.
Despite the overall decreasing trend in TB notification rate in Portugal from 2010 to 2017, we found that decline in TB notification rates was smaller in non-Portuguese nationals, HIV-negative individuals and children under five years of age (although numbers were very small for children). Most cases were notified in the three most populated Portuguese regions (Lisbon and Tagus Valley, North and Centre) but no significant differences were observed in the regional interannual declines.
Lisbon and Tagus Valley (LTV) had higher proportions of female and younger cases, non-Portuguese nationals, HIV-coinfected and extrapulmonary TB than the other six regions. These regional differences correlate well with previous evidence that had described that the most frequent risk factors for TB, and for failure to complete latent TB (LTBI) treatment, are different in LTV (cases are more frequently migrant or living with HIV) and North region (where alcohol abuse and injecting drugs seem to be more frequently associated).6-8
In Portugal, from 2015 to 2019, the TB notification rate decreased by 18.9% (from 21.2 to 17.2 cases per 100,000 population) which is close to the 20% reduction set as interim milestone at the WHO End TB Strategy for 2015–2020.2 According to this document, the reduction was expected to be of 50% for 2020–2025, 80% for 2025–2030 and 90% for 2030–2035. Sub-notification of TB, as it has been described in many countries,13,14 could play a role in the observed reduction in notification rates in Portugal – although this reduction was similar to the 19% described for the WHO European region from 2015 to 2019.15 In U.S, a country with a very low incidence of TB (under 5/100,000 population)16 there was an average decrease of only 2.1% per year in 2012–2019.
When comparing the regional declines in TB notification rates, we did not observe significant differences in Portugal. However, our results showed how those regions with higher notifications rates, mostly North and LTV, were also those with highest interannual declines. Similarly, regions with lower notifications rates (mostly Azores and Alentejo) were also the ones with lowest interannual declines.
Interannual declines in TB notification rates allow a better understanding of the evolving situation than the overall decrease in the number of cases, as the latter indicator does not consider rate increases in intermediate years within the study period. This is illustrated by the regions of Madeira, Algarve, Alentejo, Azores and LTV (Fig. 1). We believe that the interannual changes are more robust when drawing conclusions because are less affected by sporadic annual changes that can be due by a vast range of causes (including outbreaks or under-diagnosis/under-notification because of any external causes, such as the current COVID-19 pandemic).
Finally, the fact that some regions with lower incidence rates of TB at the start of the study period showed a smaller decline in notification rates could be partially explained by a smaller likelihood of receiving targeted interventions.
The results of this study showed that the declines in TB notification rates were smaller in children under five year's old and non-Portuguese nationals, although numbers were very small for children. Factors such as improved reporting and better diagnosing tools and guidelines, might have played a role. Although with some degree of uncertainty due to the small number of cases, incidence of TB in HIV-positive patients seemed to have a greater decline than in HIV-negative, which may be due to more intensive latent TB screening and preventive treatment, and to an increased antiretroviral coverage amongst HIV positive in the latest years. These results seem to be confirmed by the latest surveillance reports.
Despite the overall decline during the last years in the number of TB notifications, an increase of the total number or the relative weight of some specific groups has been described.1 Number of cases in children under 15 in Portugal and EU/EEA changed from 43 (2.0%) and 2262 (3.7%) in 2015 to 63 (3.6%) and 1995 (3.9%) in 2019, respectively. In immigrants, the number of reported cases in Portugal and EU/EEA changed from 367 to 18,543 in 2015 (16.7% and 28.2% of all TB cases reported in that year, respectively) to 419 and 17,181 (23.7% and 34.5%) in 2018, respectively. In some other risk groups, such as HIV-coinfected, the number of cases in Portugal and EU/EEA declined from 232 (11.7%) and 1248 (4.6%) in 2015 to 133 (11.0%) and 502 (3.1%) in 2019, respectively. Regarding 2019 data of extrapulmonary TB, Portugal had higher proportions than the average in the EU/EEA (26.0% vs 22.5%).1
In our study we found steeper declines in notification rates in men than in women, although differences were not significant. Men are more frequently affected by TB, which has been attributed to the fact that they present other social, behavioural and occupational risk factors for TB more frequently than women.17,18 However, recent evidence described a steeper decline in TB incidence rates in men and at older ages in the U.S., China and India.19
The main strength of this study is that it included high-quality data at national level for some of the most important risk groups for TB. The main limitation is the lack of information about other specific risk factors such as drug-using, homelessness, or incarceration. There is a need to collect information about these conditions, amongst other determinants of health.20,21 Active case finding in at-risk subgroups has been described as an effective way to strengthen TB prevention and control.22 CDC and ECDC have started analysing changes and trends in different risk groups and regions.1,16 but we still need to better monitor transmission and overlaps in these groups and obtain more precise data on the risk factors for TB as time changes.5
Other limitations include missing data, especially for HIV status (approximately 15% missing), which could have had biased our results. Our analysis includes only data until 2017, although at the time of submission more up-to-date data was available. In the context of the current COVID-19 pandemic, it has been described that most severe COVID-19 cases also have an increased prevalence of latent tuberculosis infection (LTBI).23 More research is needed to disentangle if COVID-19 could favour progression to TB or worsen outcomes and to learn more about the direct and indirect effects of the COVID-19 pandemic in TB prevention and control, including those related to social determinants of health.24,25 TB and COVID-19 have been associated with health inequities, disadvantaged socio-economic status, and poverty.26 A recent study showed lower mortality rates in immigrants diagnosed with COVID-19 and TB, although differences were attributed to the fact that migrants tend to be younger and with fewer comorbidities.27 Although it might be too soon to assess the impact that the COVID-19 pandemic may have had on the TB incidence, data from 33 centres in 18 countries have already showed a worldwide disruption of the TB services during the first four months of the pandemic.28 In Barcelona, the socioeconomic and health consequences of the COVID-19 pandemic probably had a relevant impact on TB surveillance and control in the city, where a decline of almost 20% on the number of notifications and new diagnostics was observed.29
A recent modelling study predicted long-lasting TB incidence increases in three countries with a high TB burden due to lockdown-related disruptions.14 Either way, all resources used to screen, diagnose, and treat TB or COVID-19 must be synergic in order to tackle both conditions and optimize resources.30 As it has been mentioned, the apparent success in Portugal achieving the WHO End TB Strategy milestone reduction from 2015 to 2020,2 has to be monitored to end TB by 2035. Above all, the possible under-notification due to the COVID-19 pandemic in 2020 and the likely scenario of TB incidence increase “pos-COVID-19″ must be considered.14
We recommend systematic screening of TB in migrants originating from high incidence countries, as well as raising awareness of local clinicians for the most affected population groups to consider the diagnosis of TB and ensuring that new-borns with specific risk factors are timely offered BCG vaccination. We also recommend strengthening contact tracing above all in immigrants and in settings where children are more frequently involved. Community health workers should be more frequently integrated in the response to TB, both at operational and strategical levels, to facilitate and enhance contact tracing and LTBI-TB treatment in special circumstances where idiomatic or cultural barriers exist.31
We recommend further regional-local analysis to tailor more specific interventions. We hope that the present study may also contribute to improve epidemiological surveillance, focusing on monitoring changes in risk groups and in specific geographical areas to plan prevention and control interventions more effectively, supporting the National TB Programme objective of further reducing incidence of tuberculosis in Portugal.32