Dermatomyositis (DM) is a rare disease characterized by proximal muscle weakness and a typical cutaneous rash. The muscle biopsy shows inflammatory lesions consistent with myositis, being related to an increased risk of cancer, often being considered as a paraneoplastic syndrome. The authors present a case of a 63-year-old man, with progressive proximal muscle weakness and cutaneous rash, appearing in two months. The muscle and skin biopsies were consistent with DM. Chest tomography showed that a nodular image in the lingular region and bronchy biopsy confirmed the diagnosis of small cell lung carcinoma (SCLC). This clinical case intends to enhance the importance of a thorough diagnostic study in patients with DM, as it is often a paraneoplastic syndrome.
A dermatomiosite (DM) é uma doença rara, caracterizada por fraqueza muscular proximal associada a exantema cutâneo típico. A biopsia muscular apresenta lesões inflamatórias compatíveis com miosite, estando associada a um aumento de risco de neoplasia, frequentemente considerada como síndrome paraneoplásico. Os autores apresentam um caso de um homem de 63 anos, com quadro de fraqueza muscular proximal progressiva e exantema cutâneo com 2 meses de evolução. A biopsia cutânea e muscular foram compatíveis com DM. A tomografia tórax mostrou imagem nodular paracardíaca esquerda e a biopsia brônquica confirmou diagnóstico de carcinoma pulmão pequenas células. Este caso clínico pretende realçar a importância da realização do estudo diagnóstico exaustivo em doentes com DM, visto que esta patologia surge frequentemente como síndrome paraneoplásico.
Dermatomyositis is a rare disease with an incidence of 1 per 100,000; it affects children and adults with 2:1 predominance of females. Paraneoplastic dermatomyositis most often appears after the 5th decade of life.1,3,6
Together with Polymyositis and inclusion body myositis, it belongs to the inflammatory myopathies group; progressive proximal skeletal muscle weakness, symmetric (except for the inclusion body myositis) and inflammatory infiltrates are the main clinical and histological features.3
Dermatomyositis presents characteristic skin manifestations (heliotropic rash, Gottron papules, rash on areas of sun exposure) that accompany and more often precede muscle weakness.3–5
Case reportThe patient was a male, 63 years old, Caucasian, retired stonemason. His relevant personal history included, type 2 diabetes mellitus, dyslipidemia, hypertension, obesity, ex-smoker (40 packs per year).
He had apparently been healthy until 2 months before admission, when he began to suffer from facial flushing and swelling with malar, frontal and anterior scalp papular erythema. Gradually, the skin changes become more marked and extensive, with upper and lower limb myalgia and lower limb edema.
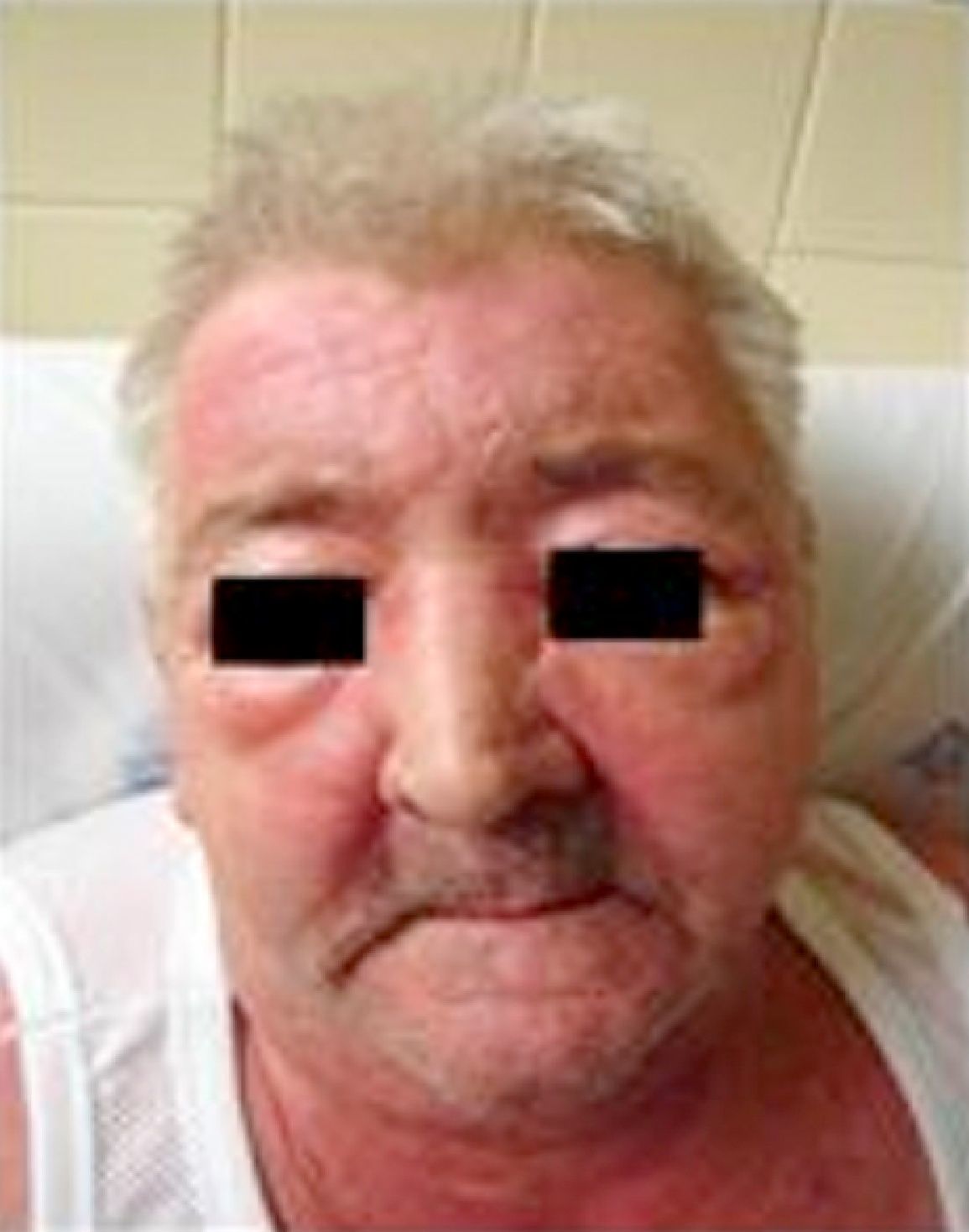
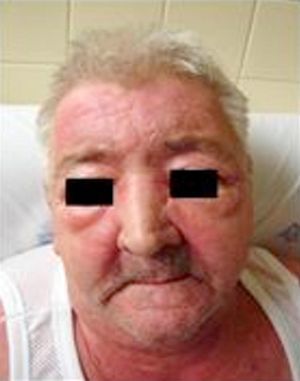
About a month after onset, the symptoms became worse and the patient went to the Emergency Room with: (1) scapular and pelvic girdles decreased muscle strength, associated with upper and lower limbs edema and stiffness (inability to get up from a chair and when climbing stairs) without changes in sensitivity; (2) MALAR and frontal erythematous maculopapular rash; (3) upper anterior, posterior and dorsal thoracic region macular rash (V-sign and shawl sign); (4) hands dorsal surface, metacarpo-phalangeal, proximal and distal interphalangeal joints erythematous maculopapular rash (Gottron papules); (5) eyelids edema and periorbital erythema (heliotropic erythema); (6) elbows and knees joints edema, with inability to straighten limbs to full extent, without erythema or increased temperature; and (7) hands and lower limbs edema (Fig. 1).
Blood tests showed LDH 810U/L (240–480U/L); GOT 151U/L (4–33U/L); GPT 46U/L (4–50U/L); CK 1073U/L (1–170U/L); myoglobin 515ng/mL (17.4–105.7ng/mL).
Given these symptoms, dermatomyositis was considered a hypothesis and the patient was admitted for further study and clarification.
During hospitalization, myoglobin and CK presented maximum values of 1037U/L and 515.5ng/mL, respectively, decreasing gradually after beginning treatment with prednisone 1mg/kg/day.
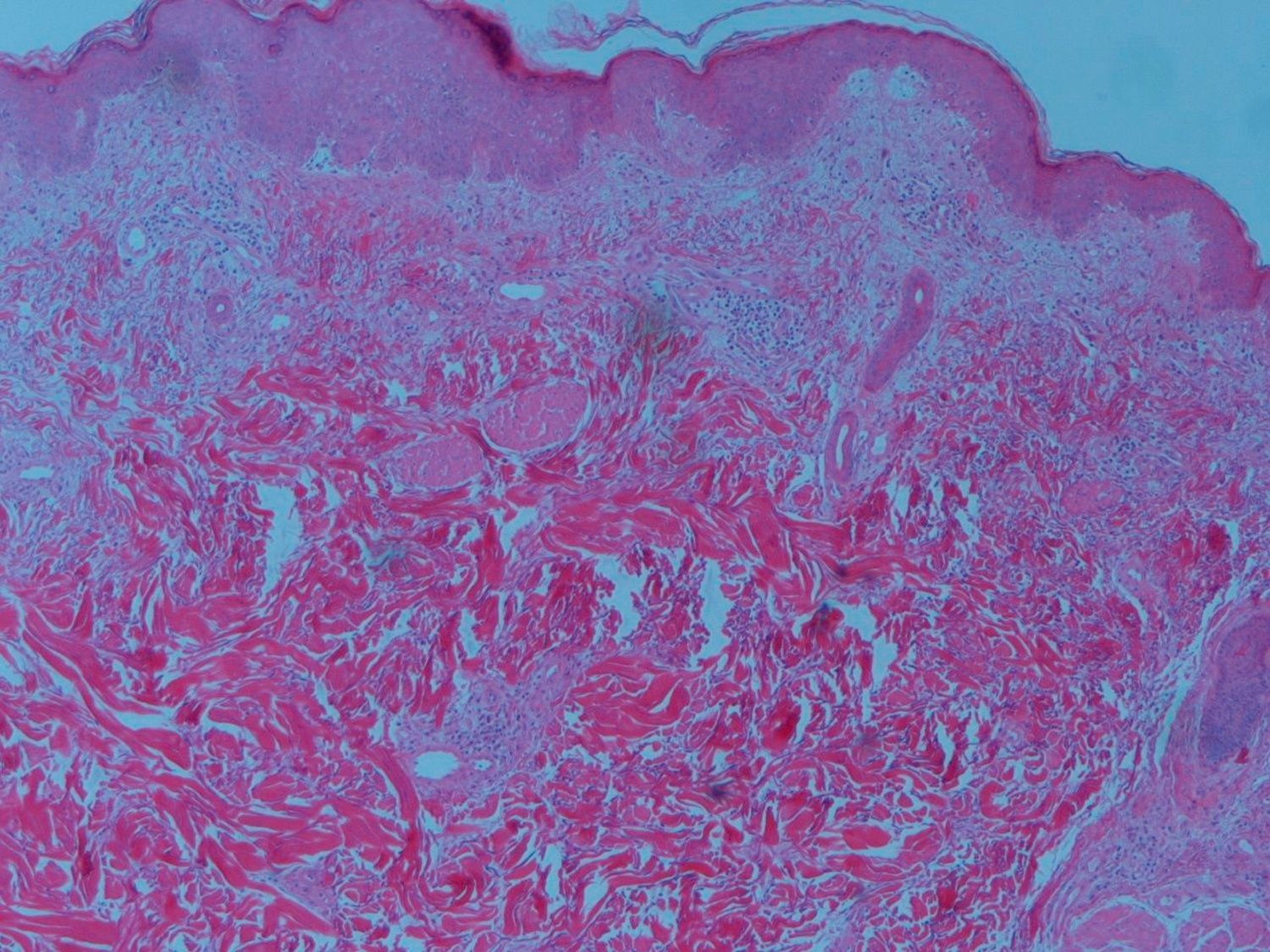
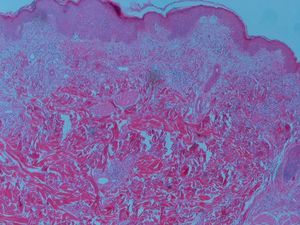
Skin biopsy revealed a mild peri-vascular patchy lymphohistiocytic infiltrate and the muscle biopsy confirmed myositis and perivascular inflammatory infiltrate, endothelial hyperplasia, perifascicular atrophy, diagnosis of dermatomyositis (Fig. 3).
The electromyography revealed a myopathic pattern consistent with myositis (short duration and low amplitude motor unit potentials, fibrillation at rest, complex repetitive and high frequency discharges), without changes in nerve conduction.
In view of the dermatomyositis diagnosis it became imperative to rule out a possible association with an autoimmune disease and/or neoplasm as these situations commonly occur.
The immunological study showed ANA 1/640 (mottled pattern), and other antinuclear autoantibodies were negative, negative myopathies autoantibodies (anti-Jo-1; Anti Ku; Anti-Pm-Scl; Anti PL-7; Anti PL-2 e Anti Ro-52).
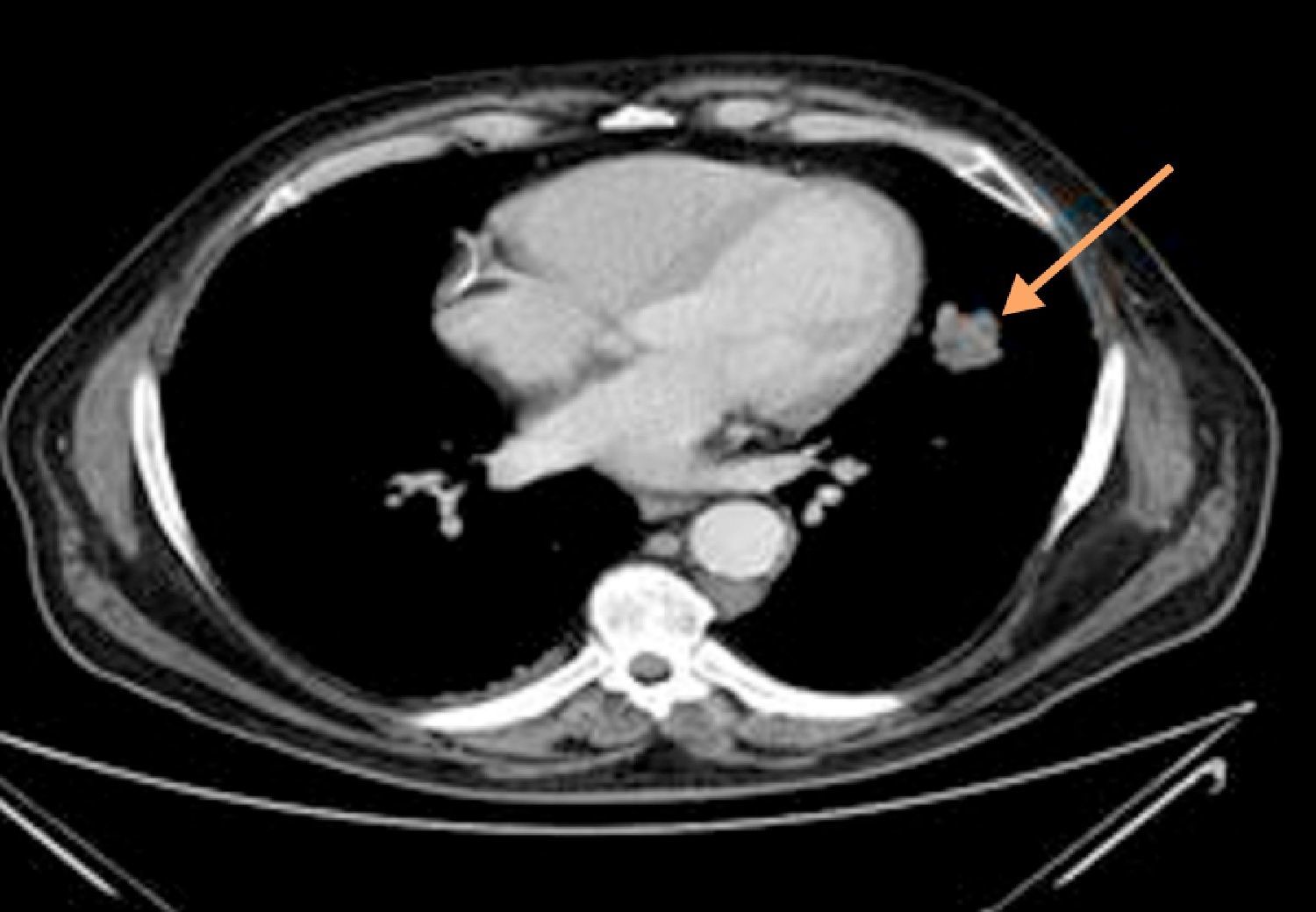
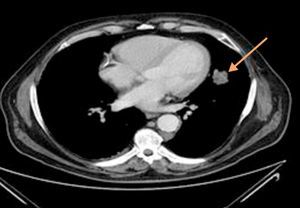
Thoracoabdominal tomography revealed a left paracardiac nodular image with 4cm×2cm diameter, with irregular limits, aspects of a probable connection to a neoplastic lesion (Fig. 2).
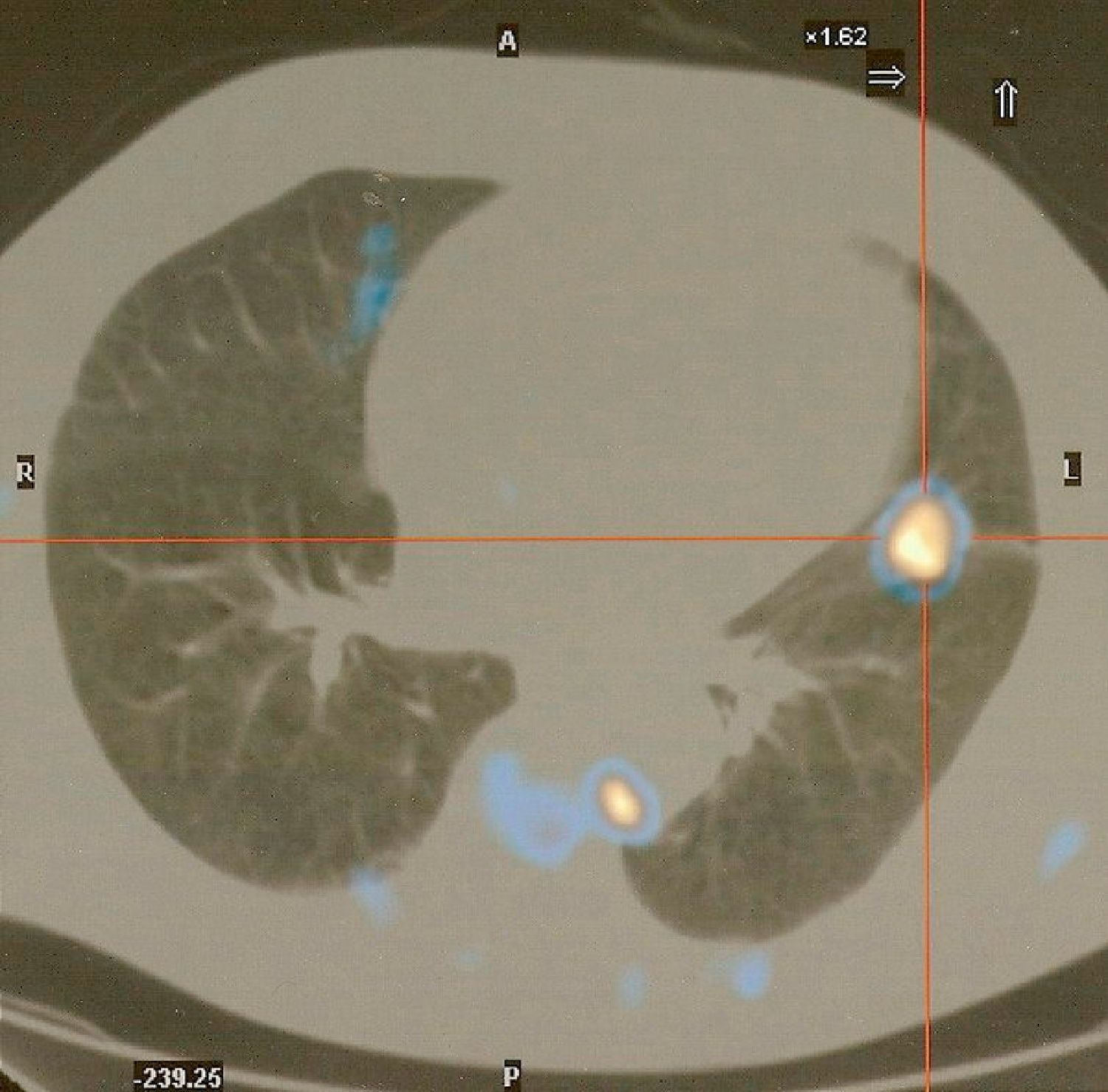
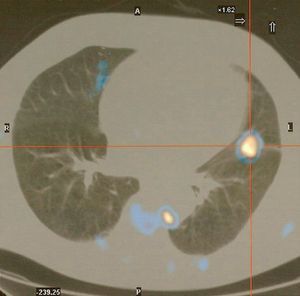
Fiberoptic bronchoscopy was performed, which besides mucosa diffuse congestion showed no visible endobronchial lesions. Biopsies and brush of B5 left segmental bronchus were guided with image intensifier. The lingular bronchial lesion mucosa biopsy confirmed an immunohistochemical diagnosis of small cell lung carcinoma (SCLC). Whole body positron emission tomography showed foci with abnormal Fluorodeoxyglucose uptake, significantly increased in the lingula; just-esophageal, left intercostal and pre-veterbral lymph nodes and also intense muscular uptake related to the inflammatory disease (dermatomyositis) (Fig. 4).
Therefore, the final diagnosis was a SCLC (T2N2M0-TNM classification) in a patient with performance status (PS) 2 and presenting as a paraneoplastic syndrome dermatomyositis.
The patient was proposed to start chemotherapy (CT). Subsequently, according to his clinical and radiological he should have CT and radiotherapy (RT) concomitantly. He underwent the first CT cycle as an inpatient, and his first febrile neutropenia occurred during that hospitalization. The remaining CT cycles also brought on serious hematological side effects (despite prophylaxis with neutrophils growth factors) accompanied by deterioration of his general condition. After 3 cycles of CT radiological evaluation showed partial remission of the disease, it was decided just to maintain CT, delaying the RT start because the patient was not strong enough (PS-2) to undergo concomitant CT and RT. After the 4th CT cycle, he was again hospitalized for febrile pancytopenia and it was decided that, given his poor general condition (PS-3) and the severity of CT side effects, the patient should only be given best supportive care. The patient was kept on systemic corticotherapy for dermatomyositis treatment from the beginning.
Eight months after diagnosis, the patient was admitted to the Emergency Department for abdominal pain and died of sepsis in the context of an acute abdomen large bowel perforation – diverticulitis.
DiscussionDermatomyositis diagnosis is confirmed when the cutaneous manifestations can also be associated the Bohan and Peter criteria that include: (1) proximal and symmetric muscle weakness; (2) serum skeletal muscle enzymes elevation (CK, aldolase, LDH, GOT and GPT); (3) myopathic changes in electromyography; and (4) muscle biopsy with myositis characteristic changes.1,2,8
There are also other symptoms which may be present in varying degrees: systemic symptoms (fever, malaise, weight loss); musculoskeletal changes (arthralgia, synovitis); dysphagia and other gastrointestinal disorders (dysmotility, malabsorption); cardiac disorders (atrial-ventricular tachyarrhythmia disorders, dilated cardiomyopathy); pulmonary changes (respiratory muscles weakness, interstitial lung disease); vascular disorders (Raynaud's phenomenon, vasculitis) and others.1,3
The disease mechanism is not yet fully understood, but the inflammatory tissue reaction, vasculitis associated with the frequent presence of autoantibodies, evidence of T cell-mediated myotoxicity or complement-mediated microangiopathy, its frequent association with other autoimmune diseases, as well as the immune response, suggests the autoimmune nature of dermatomyositis. Dermatomyositis primarily affects the endomysium capillary endothelium antigens, with subsequent complement activation, with immune complexes deposition in the capillary bed, before there are inflammatory or structural muscle changes. These events induce endothelial cell edema, vacuolization, capillary necrosis, perivascular inflammation and ischemia, with muscle fibers destruction. Atrophy reflects the characteristic perifascicular endofascicular hypoperfusion.1,3
Several autoantibodies against nuclear and cytoplasmic antigens are found in about 30% of patients with inflammatory myopathies.7 Autoantibodies against nuclear antigens (ANA, anti-dsDNA, etc.) are not myositis specific. The role of autoantibodies against RNA(tRNA) synthetase (anti-synthetase), translational factors and signal recognition particles (antibodies anti-Jo-1, anti Ku; anti-PM-Scl; anti PL-7, anti PL-2 and anti-Ro 52) is still uncertain.1,3 These antibodies occur in less than 25% of patients, and may also appear in patients with interstitial lung disease without myositis.1
Dermatomyositis is associated with an increased risk of malignancy, which is substantially greater than in other inflammatory myopathies. Diagnosis of dermatomyositis is associated with a three times higher risk of any neoplasm; the ones with increased risk are, ovary, lung, pancreas, stomach, colorectal cancer and non-Hodgkin lymphoma, whatever the histology.1,2,11
In some patients, dermatomyositis appears as a paraneoplastic syndrome, and ovary, lung and colorectal cancers are the ones most frequently involved.1,2,9,11 In these cases, dermatomyositis improves with cancer treatment and there is a recurrence of muscle weakness when the tumor relapses, thus suggesting a paraneoplastic nature. There also seem to be some clinical differences, since these patients seem to have CK values closer to normal, more often with digital vasculitis, and are less likely to have myositis-specific antibodies than patients without cancer associated dermatomyositis.2
The case described fits dermatomyositis as a lung cancer paraneoplastic syndrome. Dermatomyositis signs were the first warning, leading to the subsequent cancer diagnosis. Dermatomyositis symptoms improved with the cancer treatment, supporting their paraneoplastic relationship.
Dermatomyositis is a disease where clinical and laboratory diagnosis is relatively simple. It is essential that doctors pay close attention to the presence of typical manifestations because early treatment is so crucial to evolution and prognosis.
Investigation for occult neoplasm must be included in all examinations of patients with a dermatomyositis diagnosis, according to local epidemiology, risk factors, age and gender.2,4
In the case described we have a patient with SCLC limited to the thorax, where an improved chance of survival was expected (despite the poor overall prognosis for this histological type), since he did respond to chemotherapy. Although his cause of death was apparently unrelated to malignancy, the truth is that the general condition of the patient suffered a serious decline which prevented him from continuing the planned treatment. According to the literature10,12 the association of dermatomyositis and malignancy, advanced age, no improvement in muscle strength in the first month of treatment and deterioration of general condition are poor prognosis and mortality factors in the first year after diagnosis. These enumerated factors, with the exception of age, may explain the evolution of the disease in this particular case.
With this case report, we want to call attention to the importance of early diagnosis and timely treatment as determinants of prognosis. For this reason, a diagnosis of dermatomyositis must always be a warning sign in medicine.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Castro AS, et al. Dermatomiosite como primeira manifestação de uma neoplasia pulmonar. Rev Port Pneumol. 2013. http://dx.doi.org/10.1016/j.rppneu.2012.11.002.