We reviewed the most important diagnostic procedures implemented by means of flexible bronchoscopy, including bronchoalveolar lavage, bronchial brushing and biopsy, transbronchial lung biopsy and transbronchial needle aspiration.
We reviewed the tools, techniques and potential complications of this examination.
Foram revistos os mais importantes procedimentos diagnósticos aplicados através de broncoscopia flexível, nomeadamente o lavado broncoalveolar, a biopsia e o escovado brônquico, a biopsia pulmonar transbrônquica e a punção aspirativa transbrônquica.
Foram revistos os instrumentos necessários, as técnicas e as possíveis complicações deste exame.
Flexible bronchoscopy has been and still is a useful diagnostic technique since its introduction by Shigeto Ikeda1 in the late sixties. Several tools and procedures have been developed for specific purposes to obtain different kinds of biological products for diagnosis in pulmonary pathology.
Due to its widespread use during the last forty years it is now an indispensable tool for diagnosis and as well as therapeutics.
In combination with ultrasound, specific lighting and different kind of probes, the utility of flexible bronchoscopy will continue to expand in the future.
In this article a review of flexible bronchoscopy with all the ancillary procedures and tools will be presented.
The toolsFlexible bronchoscopes are available in different sizes allowing the observation of the bronchial tree to the level of the fifth generation of bronchi. Image quality has been greatly improved with the new generation of videobronchoscopes and specific additional capacities such as narrow band imaging,2 autofluorescence3 or even magnification bronchoscopes.4
However, to perform the various bronchoscopic diagnostic techniques, collector devices, syringes, brushes, biopsy forceps and needles of various types are available.
In recent years new tools such as miniprobe ultrasound,5 endobronchial ultrasound bronchoscopes (EBUS)6 or navigation systems,7 which allow a better orientation of the instruments, have been introduced. Confocal fluorescence laser micro-endoscopy8 and optical coherence tomography bronchoscopes9 are both still in research phase.
Although widely known and used by all those who perform bronchoscopy, it is important to highlight some specific aspects of each instrument. Bronchoscopists should all be familiar with the instruments they have at their disposal.
Collectors’ devices, of which there are several models, should be easy to use and able to recover sufficient amount of bronchial lavage for cytological or bacteriological tests.
The syringes for bronchoalveolar lavage in the adult should have between 20 and 60ml of capacity.
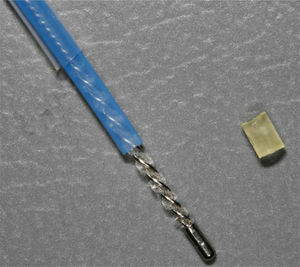
Brushes for exfoliative cytology are usually sterile, single use and retractable from an outer sheath, available in different sizes, stiffness and flexibility (Fig. 1).
For microbiologic propose the protected specimen brush (PSB) is contained in a protective sheath and covered by a biodegradable plug, thus avoiding contamination during passage through the working channel (Fig. 2).
There are three basic types of forceps, those with serrated edge (alligator), smooth edge with fenestrated or unfenestrated cups and ‘spiked’ (needle between the cups). The forceps may have round (shorter) or oval (longer) blades; some with asymmetric opening system of the blades, others for the specific purpose of multiple biopsies at the same time and even others that allow rotation of the blades for better approach of specific anatomic lesions and locations.
Forceps can be reusable or single use. Forceps manufacturers may use labels − numerical and / or colours − to distinguish their features and diameter, which allows us to choose which is most suitable for each situation and more appropriate for the diameter of the bronchoscope operator channel.
Cryoprobes, hot biopsy forceps or electrocautery snares can also be used to obtain biopsy material.
A variety of needles are available for transbronchial needle aspiration (TBNA). Needles with 13 to 15mm long and 20- to 22-gauge are used usually to obtain cytology specimens and 19-gauge to obtain a core of tissue for histology. For central lesions, it is best to use a stiffer needle to allow exertion of enough force to push the needle through the tracheobronchial wall into the lymph node. For peripheral lesions, a more flexible catheter is desirable. The coaxial needles slid inside a flexible catheter that has a proximal end to which suction can be applied and are controlled by a proximal device to handling the set.
Biopsy needles are also available as a dual needle system involving 21- and 19-gauge bevelled, retractable needles.
The needles for EBUS-TBNA bronchoscope have specific features: 21 or 22 gauge; the surface of the needle tip with a dimpled echogenic design to improve visibility on ultrasound images; two-way adjustable safety handle to protect bronchoscope channel; adjustable needle length from 20mm to 40mm.
The proceduresBronchial washingThe bronchial lavage is commonly used along with other diagnostic techniques. It consists in the instillation of 5 to 30ml of saline, frequently, in order to clear secretions or mucus in the area that will be biopsied. The fluid is then aspirated into a collector and used for cytological and microbiological studies. Ten millimetres are needed for each study. The microbiological study of bronchial lavage is always hampered by contamination of the respiratory flora.
Regarding the cytological study for diagnosis of cancer, several authors studied the sequence of washing over other procedures on diagnostic yield. Authors found that bronchial washing in relation to other sampling techniques for diagnosing bronchial tumours does not influence the diagnostic yield10,11 but “although the additional diagnostic yield of washing and brushing during bronchoscopy is relatively low, it is cost-effective to use these procedures in the diagnostic workup of patients who are clinically suspected of having a pulmonary malignancy”.12 According with the British Thoracic Society guidelines on diagnostic flexible bronchoscopy, a combination of biopsy material, brushings, and washings should be obtained, although the number of tumours diagnosed by washings alone is relatively small.13
Bronchoalveolar lavageBronchoalveolar lavage (BAL) is a minimally invasive technique indicated in every patient with interstitial lung disease.
In diffuse lung disease the middle lobe or lingula are the standard sites for BAL. In patients with radiographic heterogeneity, fluid, usually sterile isotonic saline (0.9% NaCl) solution, can be instilled into two or three different regions, to reach a higher representation of the entire lung. The bronchoscope is placed in wedge position at the level of the third- to fourth-order bronchi and the, fluid slowly instilled with syringes through the biopsy channel. Fluid retrieval should be gentle and with constant suction to minimizing bronchial collapse. A standard number of 20–60mL aliquots up to a total volume of 100–300mL in adults must be used. Usually, 40–70% of the instilled volume is recovered.14 Prior warming of the fluid to body temperature may decrease coughing.
BAL has a specific value for the diagnosis of several rare diffuse lung diseases, including alveolar proteinosis, lipoid pneumonia, acute eosinophilic pneumonia, pulmonary lymphoma and lymphangitic carcinomatosis.
BAL is essential in excluding or confirming diffuse alveolar haemorrhage in patients with unexplained pulmonary infiltrates. The diagnosis of opportunistic lung infection, like Pneumocystis pneumonia often requires the performance of BAL.
In fibrosing lung disorders, a BAL neutrophilia, often associated with BAL eosinophilia, is common. BAL lymphocytosis, with a variable BAL neutrophil content is very characteristic of granulomatous disease. Absence of BAL lymphocytosis is useful in excluding hypersensitivity pneumonitis or sarcoidosis.
In suspected drug-induced lung disease, BAL eosinophilia usefully increases the diagnostic likelihood, as does the presence of foamy alveolar macrophages in amiodarone-induced lung disease.15
Bronchial brushingBronchial brushings can be used for exfoliative cytology or microbiological analysis. The technique is similar for both purposes. The brush should be kept within its protective sheath while it is passed through the working bronchoscopic channel, then directed to the target site while an assistant advances the brush out approximately 3cm from the bronchoscope. Back and forth as well as rotating movements can all be done at the same time over the visible lesion. The brush in then retracted into its sheath and pulled back through the working channel. Distal bronchial brushings are usually performed with fluoroscopic guidance to localize the lesion, avoiding contact with the pleura and preventing complications such as pneumothorax. Bronchial brushings for exfoliative cytology use disposable cytology brush, with a diagnostic yield of 72% and 45%, respectively, for central and peripheral lesions.16
Several factors may influence the brushing efficacy. Michaelson et al. examined the cellular yield expressed as the number of cells recovered with two different types of sheathed cytology brushes. Longer and wider bristles seem to provided greater yield in total number of cells recovered per brush.17 Furthermore, Hansen and al. using sheathed brushes with 1, 1.7 and 3mm diameter showed no significant difference in cell recovery among the three brushes.18 Sato et al. state that, the diameter of the bristles may also influence the yield of brushing, according with a study in pigs.19 Popp et al. performed a study in patients with lung tumours, where brushing was repeated until five times under guidance of radiographic fluoroscopy. The sensitivity for diagnosis increased from the first to the fourth brushing and was better in central than peripheral malignant tumours. Authors concluded that repeated brushing should be done to obtain a higher diagnostic yield.20
In the author's personal experience using 1mm stiffer brush with a mix technique of back and forth movements along with rotation of the brush works perfectly in more proximal lesions. In more distal lesions a softer 3mm brush allows easier rotation while preventing lung damage.
For protected bacterial sampling a double sheath catheter should be used, advancing the outer sheath 3cm beyond the distal tip of the bronchoscope into the airway and then push the inner sheath containing the brush. The gel protection is then removed by pressing with the brush, allowing the sampling by using the same movements as with cytological brushing. After that, the brush is retracted into the inner sheath, which in turn is retracted into the outer sheath and then removed from the bronchoscope. After wiping the distal tip of the catheter with 70% alcohol, the brush is advanced and the wire cut with a sterile scissor, into at least 1mL of nonbacteriostatic saline container.21
Bronchial biopsyBronchial forceps biopsies can be performed in two main settings: biopsy of a visible bronchial malignant or benign lesion and biopsy of more distal lesions, not visible endobronchially.
For a correct bronchial biopsy under direct vision cleaning of blood or mucus covering the lesion is desired. The forceps should only be inserted through the operating channel of the bronchoscope with the tip in neutral position to avoid internal damage of the operating channel. After the distal end of the biopsy forceps exits the distal end of the bronchoscope, it should be fully opened and rotated for the best approach of the target area and then, pushed against the lesion. Once adequately anchored, the forceps is then closed and together the bronchoscope and forceps should be gently pulled under direct vision. Only then should biopsy forceps be pulled-out of the bronchoscope. The procedure is the repeated for extended or deeper sampling according to the needs.
In our Department sometimes we use a slight variant technique in which, after closing the forceps, it is pushed distally along with the bronchoscope, stripping longer and wider biopsy fragments – push biopsy.
Lesions along the tracheal wall need a more angular approach or the use of a forceps with an anchor needle. Alligator forceps are preferable in harder or cartilaginous lesions.
At least five biopsies at the same location should be taken to maximize the chance of obtaining histological evidence of neoplasia.22
Schreiber et al. have done a systematic search of literature databases until July 2001 including thirty studies of patients with central cancer lesions. Endobronchial biopsies provided the highest sensitivity (0.74; 20 studies), followed by brushings (0.59; 18 studies) and washings (0.48; 12 studies). The overall sensitivity for all bronchoscopic modalities combined, where reported, was 0.88 for centrally located endobronchial disease (14 studies).23
The diagnostic yield of bronchial brushing combined with biopsy gives higher percentage of positive diagnoses,24 and it seems better to do brushing after biopsy.25
New approaches for diagnosing endobronchial lesions using hot biopsy forceps or cryobiopsy have been reported in the literature.
Tremblay et al. performed a study to determine the impact of electrocoagulation forceps (Hot biopsy) when compared with conventional (Cold biopsy), on pathological diagnosis and also in the bleeding rates. Concordance between hot and cold samples was 92.5% for the clinical pathologist and 87% for the blinded pathologist, with minimal electrocoagulation damage. Biopsy with electrocoagulation forceps showed a reduction in the number of mild bleeding episodes (bleeding not requiring intervention) but not in moderate bleeding episodes (bleeding requiring ice-cold saline, topical adrenaline or electrocoagulation).26
Khana et al. carried out a randomized study of hot biopsy versus conventional biopsy in the diagnosis of endobronchial lesions and concluded that hot biopsy does not alter specimen quality but does not result in any significant reduction in procedure-related bleeding.27
Cryotherapy has been used in endobronchial treatment for years. New cryoprobe with greater stability to traction was built and is now available. Schumann, Hetzel et al. performed a study to evaluate the diagnostic yield and safety of cryobiopsy when compared with conventional forceps biopsy. During six years, 296 patients with visible endoluminal tumor lesions were included in the study. Authors compared both techniques in the first 55 cases and concluded that cryobiopsy is safe, feasible in routine bronchoscopy and increases the diagnostic yield in endobronchial tumor lesions.28 Another study designed by Aktas et al. to evaluate the efficacy of cryobiopsies in histopathological diagnosis has been done. Authors used three forceps biopsies followed by one cryobiopsy in 41 patients with exophytic endobronchial lesions and obtained larger samples with cryoprobe, similar haemorrhage complications in both techniques and more successful diagnostic results with cryoprobe.29 Recently, a prospective, randomized, single blinded multi-centre study to evaluate cryobiopsy over conventional endobronchial sampling was done. A total of 593 patients were randomized in 8 centres. In 563 patients malignant disease was diagnosed. Diagnostic bronchoscopy was done in 281 patients using conventional forceps and in 282 using cryoprobe. Diagnostic yield for cryobiopsy was significantly higher however bleeding was also more frequent in this group. Mild bleeding (no intervention need for control) was significantly higher in cryobiopsy, but more severe bleeding, needing intervention to control, was similar in both groups.30
Transbronchial lung biopsyIn the case of localized peripheral lung lesions, TBLB is usually performed under fluoroscopic guidance, but it seems to be unnecessary in patients with diffuse lung disease.5
To perform TBLB the bronchoscope is advanced as far as it can go and maintained in the wedged position while the biopsy forceps is inserted into the working channel. After the exact point is identified, the biopsy forceps is gently pushed to the lung periphery and opened 5-6mm proximal to the area to be biopsied. The biopsy forceps is advanced to the lesion and then closed. If the patient feels pain at this time, it is preferable to withdraw the forceps and choose another point to biopsy. After biopsy forceps removal, the bronchoscope must be maintained in the wedge position to detect or control any bleeding and keep the best position for the next biopsy.
For peripheral pulmonary lesions TBLB has a sensitivity that varies according to the number of biopsy specimens taken and to the size of the lesion. Seven to eight transbronchial biopsy samples have been proposed.31
An improvement of diagnosis yield has been shown using ultrasound-guided biopsy32,33 or electromagnetic navigation.7 Radial transducer ultrasound miniprobes can be introduced through a standard bronchoscope biopsy channel to perform EBUS.
These radial ultrasound miniprobes, in combination with fluoroscopy, can guide a sheath to assess target peripheral lesions. Once reached the lesion, the probe can be withdrawn with the guided-sheath left in place, acting as an extended working channel. This allows the passage of tools, like biopsy forceps or brush, into the sub segmental bronchus of interest and histopathology specimens can be obtained.7,31
TBLB is performed in the diagnostic work-up of diffuse lung infiltrates, with a yield in the order of 75%.34 Four to six transbronchial biopsy samples should be obtained from one lung.35 The type of forceps used (alligator versus cup) does not seem to influence the diagnostic yield,36,37 however cup forceps are more likely to obtain the desired alveolar specimen.38
As for bronchial biopsies, transbronchial lung cryobiopsy was purposed by Babiak and Hetzel J. In a study of forty one patients with diffuse lung disease, transbronchial lung cryobiopsy under fluoroscopic guidance, the authors concluded that this technique obtained larger and better lung biopsy samples than that with usual forceps biopsy.39
Transbronchial needle aspirationTBNA is a technique for sampling mediastinal structures adjacent to the tracheobronchial tree as well as lesions within the airway wall or in lung parenchyma.40 TBNA is the only technique that allows sampling tissue from beyond the endobronchial tree, such as enlarged lymph nodes, peribronchial or submucosal lesions. It is useful for cytological, histological or microbiological analysis.
TBNA with flexible bronchoscopy was developed in the late 1970s by Wang. He also designed specific needles for cytology that are still used nowadays.41 Later in 1990s Wang developed needles for histologic purposes, which became a landmark in this technique.42
The TBNA technique for cytology or histology is similar and was well described by Dasgupta and Mehta. The needle can be inserted through the bronchial wall through four techniques: in the Jabbing method, the needle is thrust through the intercartilaginous space with a quick, firm jab to the catheter while the scope is fixed at the nose or the mouth. In the Piggyback method, once the needle is advanced and locked into position, the catheter is fixed against the proximal end of the insertion port using one finger. The bronchoscope and catheter are then pushed forward as a single unit until the entire needle penetrates the tracheobronchial wall. In the cough method, the Jabbing or pushing (Piggyback) technique is applied while the patient coughs to facilitate penetration of the needle. In the Hub against the wall method, with the needle retracted, the distal end of the catheter can be placed directly in contact with the target and held firmly while the needle is pushed through the tracheobronchial wall. All of these techniques can be used singly or in combination.43
After the airway wall penetration, suction is applied using a syringe. When the vacuum in the syringe is confirmed, rapid to and fro movements with the catheter are performed. After the suction is released, the needle is pulled out. The tip of the bronchoscope is straightened, and the catheter is then withdrawn from the scope in a single smooth motion. Releasing suction before withdrawing the needle prevents false-positive results.43
A dedicated endobronchial ultrasound (EBUS) − TBNA bronchoscope is available since 2004. Endobronchial ultrasound is a minimally invasive technique that has found its application in the evaluation of mediastinal lymphadenopathy, intrapulmonary tumours and lung cancer staging. EBUS allows visualisation of the tracheobronchial wall and adjacent structures.44,45
After establishing the presence of enlarged lymph nodes and identifying adjacent structures, the bronchoscope is placed in position and the needle is introduced through the working channel in a retracted position. Subsequent TBNA is done in a conventional way.44
EBUS-TBNA allows real-time guided sampling of intrathoracic lymph nodes, and has the advantage of identifying vascular structures and minimizing risks.
TBNA or EBUS-TBNA diagnostic yield can be improved by a rapid on-site cytological evaluation (ROSE) (Figs. 3 and 4), but this technique is underused. ROSE allows you to confirm, at the bronchoscopy suite, the achievement of suitable material or even a provisional diagnosis. Increasing the use of on-site cytopathology assessment may improve the quality of bronchoscopy services and is cost-effective.46–48
To determine the number of aspirates need in a TBNA bronchoscopy Chin et al. studied the number of aspirates performed during flexible bronchoscopy in patients with known or suspected lung cancer and enlarged mediastinal lymph nodes. Their data indicate that a high yield occurs with the first puncture, but an increment in yield with successive aspirates until seven was seen. In diagnostic aspirates, 93% of them were obtained in the first four attempts and only more 7% when going to the seventh specimen. Authors concluded that at least four TBNAs in a single node station should be attempted to obtain adequate material, but seven passes will maximize yield.49
Current lung cancer staging guidelines advocate oesophageal endoscopic curved-linear ultrasound with fine-needle aspiration (EUS-FNA) and EBUS-TBNA as an alternative for surgical staging.50,51 EBUS-TBNA allows to achieve lymph nodes station 2 (upper paratracheal), 4 (lower paratracheal), 7 (subcarinal),10 (hilar) and 11(interlobar) in right and left side, as described on the new International Association for the Study of lung Cancer node map.52,53 Station 8 and 9 are only achieved by EUS-FNA.53
However, the recent British Thoracic Society guidelines for advanced diagnostic and therapeutic flexible bronchoscopy in adults recommend the use of conventional TBNA to sample mediastinal and hilar lymphadenopathy at initial diagnostic bronchoscopy where a pre-procedure chest computed tomography (CT) has demonstrated enlarged lymph nodes, and real-time EBUS-TBNA should be considered in a non-diagnostic conventional TBNA.54
Dooms et al. also recommend the use of conventional TBNA during the initial flexible bronchoscopy if enlarged mediastinal lymph nodes are seen on chest CT. Lymph nodes with clear anatomic landmarks (such as lower right and left paratracheal mediastinal lymph nodes (position 4R and 4L respectively), as well as the subcarinal lymph nodes (position 7) and hilar lymph nodes (position 11), which are clearly enlarged − largest short axis of 15mm − can be adequately sampled.55
Beyond staging lung cancer, TBNA can also be used as an additional procedure to diagnose endobronchial lesions. The addition of TBNA to biopsy or brushing may increase the diagnostic yield. Dasgupta et al. designed a prospective study comparing the yield of conventional diagnostic procedures with or without TBNA. In their series diagnostic yield of 65% with conventional procedures, compared with the 96% yield obtained by the addition of TBNA. A similar increase in diagnostic yield was observed in exophytic mass lesion when TBNA was added to biopsy and brushing. Although this increase did not reach statistical significance, it diagnosed lung cancer in four additional patients. The authors concluded that the potential for TBNA to bypass surface necrosis and sample viable tumor from deep within the mass is a possible explanation. In extrinsic compression, conventional procedures using brushing or biopsy tend to sample mainly the surface rather than deep within the lesion. In these situations, the ability of TBNA to provide adequate sampling by penetrating either the submucosa or directly through the bronchial wall into the tumor mass could enhance diagnostic yield.56 These results were confirmed by other authors.57,58
Several authors support the use of TBNA in conjunction with TBLB in all patients with stage I and II sarcoidosis, since it markedly increases the diagnostic yield of bronchoscopy.59–61 The recent British Thoracic Society guidelines for advanced diagnostic and therapeutic flexible bronchoscopy in adults recommend “conventional TBNA is a safe technique for sampling hilar and mediastinal lymph nodes in cases of suspected sarcoidosis and may be used in conjunction with endobronchial and transbronchial biopsies.54
The complicationsFlexible bronchoscopy is a safe procedure. Large retrospective studies on mortality and morbidity were published more than 25 years ago describing mortality rates under 0.04%, with a major complication rate between 0.08% and 0.3%.62,63 A more recent retrospective study with over 4000 cases, showed no deaths and overall major complication rates of 0.5%.64
Major complications were defined as death, cardiopulmonary arrest, myocardial infarction, pneumonia, pneumothorax (larger than 20 percent or requiring chest drainage), airways obstruction severe enough to require therapy, pulmonary haemorrhage requiring transfusion, seizures and stroke. Minor complications were defined as bleeding in amounts greater than 50ml but not requiring transfusion, pneumothorax smaller than 20 percent, vasovagal reaction, mild airways obstruction, arrhythmia, and a miscellaneous group of complications.65
Before proceeding with a bronchoscopy we must be aware that some patients have a higher potential for complications. Risk versus benefit ratio should be carefully analysed in patients with life-threatening arrhythmias, myocardial infarction in the preceding 4 to 6 weeks, unstable angina, persistent hypoxemia, thrombocytopenia under 50.000/μl, coagulopathies, severe uraemia, superior vena cava syndrome, pulmonary hypertension and those that are uncooperative.16
Infection seems to be rare after bronchoscopy however a bacteraemia has been reported in a 6.5% rate. Prophylactic antibiotics are not required except in asplenic patients and in those who have a prosthetic valve or a previous history of endocarditis.13
Proximal brushing and bronchial biopsy are usually related with minimal bronchial haemorrhage and most of the times, additional measures to control bleeding are not needed. Distal brushing is rarely associated with pneumothorax and the risk can be minimized with the use of fluoroscopy.
LBA is associated with practically no mortality and carries a low complication rate of 0–2.3%. Fever and a transient decrease in lung function parameters, both usually self-limited to 24h, occur in 3–30% of patients, depending on the instilled volume. Wheezing and bronchospasm in patients with hyperreactive airways may happen. Major complications are only seen in patients with severe lung or heart disease, and bleeding has only been reported in patients with clotting disorders or thrombocytopenia.14
Complication rates related to transbronchial lung biopsies are higher. Pneumothorax occurred in less than 5% of cases and haemorrhage in 9%, which was usually mild. Risk factors for bleeding include uraemia, liver failure, immunosuppression, pulmonary hypertension and coagulation disorders. Overall mortality has been reported as 0.1%.13 Major complications occurred in 6.8% in another series.64
Complications related to EBUS-TBNA are similar to those of conventional TBNA, including bleeding from major vessels, pneumomediastinum, mediastinitis, pneumothorax, bronchospasm and laryngospasm. Although reported complication rates are very low, Varela-Lema et al. performed a systematic literature review of real time EBUS–TBNA carried out until November 2007 and updated in April 2008. Twenty publications were included and none of the studies reported serious complications. Only three studies reported having observed agitation, cough, and presence of blood at the puncture site.66 In over 2000 cases Yasufuku et al. found no complications related to EBUS-TBNA besides damage of the bronchoscope.67
However, more recently, Zabaleta reports one case of infectious complications after EBUS-TBNA, pointing out more cases described in literature and warning that this complication demands early diagnosis and treatment.68
ConclusionsFlexible bronchoscopy is a widely available technique since its introduction in the late sixties with great impact on pulmonary medicine. It represents a major advance in the diagnosis and management of lung diseases. Several ancillary procedures have been developed for specific purposes to obtaining different kinds of biological products for pulmonary diagnosis.
Over the years some new tools have emerged, like conventional transbronchial needle, which has allowed the possibility of sampling through the central airways wall.
In more recent years, new ideas have become available, which are especially addressed to mediastinal lymph nodes staging and diagnosis of peripheral lesions in the lung.
EBUS-TBNA dedicated bronchoscope has been used as a standard procedure because of its improved diagnostic yield in mediastinal diagnosis or staging. However, the recent British Thoracic Society guidelines recommend the use of conventional TBNA to sample mediastinal and hilar lymphadenopathy at initial diagnostic bronchoscopy where appropriate.
Bronchoscopists face numerous challenges to establish the correct diagnosis of peripheral lung diseases. Some diagnostic bronchoscopies fail to achieve the designated goals, so patients have to repeat bronchoscopy or need additional procedures. The use of ultrasound probes or electromagnetic navigation allow a more accurate approach of peripheral lesions.
In the near future new diagnostic techniques such as fluorescence confocal laser micro-endoscopy and optical coherence tomography bronchoscopes will reach a suspicious area and make an optical biopsy. These techniques, however, still require further research.
Conflicts of interestThe authors have no conflicts of interest to declare.