Patients with idiopathic pulmonary fibrosis (IPF) present respiratory derangements at rest and during exercise, accompanied by exercise intolerance. Some patients may develop profound exertional desaturation even without resting hypoxemia. Evidence suggests the involvement of reduced cerebral-oxygenation in exercise intolerance. We aimed to examine (i) differences in cerebral-oxygenation during exercise between IPF patients with and without isolated exertional desaturation, (ii) whether the impairments in cerebral-oxygenation are detected at similar exercise intensity, and (iii) correlations between cerebral-oxygenation indices, disease severity, and 6-min walk test (6MWT).
Materials and MethodsPatients with IPF (n = 24; 62.1 ± 9.3 years) without resting hypoxemia underwent cardiopulmonary exercise testing (CPET) with cerebral-oxygenation monitoring via near-infrared-spectroscopy (NIRS). Βased on their pulse-oxymetry saturation (SpO2) during CPET, patients were divided into the “exertional-desaturators” group (SpO2nadir≤89% and ≥6% drop in SpO2) and the “non-exertional-desaturators” group (SpO2nadir≥90% and ≤5% drop).
ResultsDuring CPET, the “exertional-desaturators” group exhibited lower oxygenated-hemoglobin (-0.67 ± 1.48 vs. 0.69 ± 1.75 μmol/l; p < 0.05) and higher deoxygenated-hemoglobin (1.67 ± 1.13 vs. 0.17 ± 0.62 μmol/l; p < 0.001) than the “non-exertional-desaturators” group. A different pattern (p < 0.01) in cerebral-oxygenation responses was observed in the two groups. In exertional-desaturators oxygenated-hemoglobin declined below baseline even at low/moderate-intensity exercise (p < 0.05), whereas, in non-exertional-desaturators cerebral-oxygenation declined (p < 0.05) at high-intensity exercise. Cerebral-NIRS indices correlated (p < 0.05) with CPET-duration, dyspnea, diffusion capacity, and 6MWT.
ConclusionsDuring incremental exercise, patients with IPF and exertional desaturation present a significant decline in cerebral-oxygenation even during low-intensity exercise. Our findings support the implementation of longer-duration rehabilitation programs in IPF so that lower intensity exercise can be applied at the initial stages. (NCT 03683082)
Idiopathic Pulmonary Fibrosis (IPF) is a chronic progressive disorder characterized by aberrant accumulation of fibrotic tissue in the lungs, associated with poor prognosis.1 In concert with alterations in the mechanical properties of the lungs and abnormalities in the lung vasculature in IPF, impairments in ventilation/perfusion occur, and the lung's diffusing capacity is progressively reduced.2,3 The multiple derangements of the lung physiology in IPF, translate into significant reductions in exercise capacity and development of dyspnea.4–6 Some patients may develop profound exercise-induced desaturation, even when oxygen saturation is acceptable when at rest.2,6 Importantly, exertional desaturation and abnormal ventilatory responses during exercise are strong predictors of mortality in IPF.7–9 Pathophysiologic mechanisms of exertional desaturation include ventilatory-perfusion mismatching, lung diffusion limitations, low mixed venous oxygen concentration, pulmonary vasculopathy, and high tissue oxygen extraction during exercise.6,10–12 Exertional dyspnea, another cardinal feature of IPF,13 also appears multifactorial, as abnormal pulmonary mechanics, hypoxemia, impaired cardiocirculatory responses, and peripheral muscle dysfunction contribute to its establishment.9,14,15 Hypoxia and hyperventilation during exercise can reduce cerebral blood flow and also limit exercise tolerance,16 however, studies examining the role of cerebral-oxygenation in exercise intolerance in IPF are limited.
In healthy individuals, cerebral-oxygenation during exercise increases from low to moderate/high intensities (25–75% of peak oxygen uptake, VO2peak) and decreases at peak/exhaustive exercise.17 Decrements in cerebral oxygenation can reduce cortical activation, contributing to the development of central fatigue, decreasing the muscles’ force-generating capacity, and resulting in exercise termination.18,19 A blunted cerebral-oxygenation during exercise has been reported in various chronic diseases,20–22 and in patients with COPD it has been associated with exertional-dyspnea.23 To the best of our knowledge, only two recent studies examined brain-oxygenation during exercise in patients with IPF/interstitial lung disease (ILD). Specifically, Marillier et al.24 showed greater cerebral-desaturation during exercise in patients with ILD vs healthy controls; authors reported that cerebral responses varied substantially across patients. Patients with ILD were then divided based on the presence of cerebral-deoxygenation; an association of cerebral-deoxygenation with lower peak oxygen uptake (VO2peak) and hypoxemia was observed.24 The second study,25 showed that oxygen-supplementation during steady-state exercise (at 65% of VO2peak) improved brain/skeletal muscle-oxygenation, and prolonged exercise duration in patients with IPF. Although similarities in exercise-induced hypoxemia are documented in patients with ILDs of various aetiologies, there are differences in the pattern and gas exchange severity during exercise between IPF and other ILDs, attributable to specific underlying pathophysiological mechanisms.11 Whether patients with IPF and isolated exertional desaturation, present lower cerebral-oxygenation during incremental exercise compared to patients without exertional desaturation and whether greater gas exchange abnormalities during exercise in the former group translate into the development of cerebral-deoxygenation at lower exercise intensity (lower VO2peak percentage), have not been examined. This question is of importance for pulmonary rehabilitation professionals, in order to choose the appropriate exercise intensity for patients with IPF with and without exertional desaturation and avoid significant reductions in cerebral-oxygenation, possible negative effects on cognitive function/performance,26 and premature exercise termination. Thus, the primary aims of this prospective, case-control study were to (i) examine whether patients with IPF and isolated exertional desaturation (as assessed by pulse oximetry, SpO2) present lower cerebral-oxygenation during exercise vs those without exertional desaturation and (ii) identify whether decrements in cerebral-oxygenation occur at similar exercise intensity (%VO2peak) in both groups. Secondary aims were to (i) explore the correlation of cerebral-indices during cardiopulmonary exercise (CPET) with exercise duration, dyspnea, lung diffusion capacity, and 6-min walk test (6MWT) in patients with IPF without resting hypoxemia.
Materials and methodsParticipantsPatients with IPF (diagnosed based on current ATS/ERS guidelines2) were recruited from the ILD outpatient clinic, Papanikolaou Hospital (9/2018-2/2020). Inclusion criteria: (i) Patients stable on IPF medication, without hospitalization due to respiratory failure/infection during the past three months, (ii) resting arterial oxygen tension (PaO2)≥60 mmHg and systolic pulmonary artery pressure (sPAP)<25mmHg. Exclusion criteria: (i) Patients with pulmonary hypertension or absolute contraindications for maximal CPET,27 (ii) patients with SpO2≤94 while sitting quietly in room air. Participants were divided into two groups: a) patients with exertional desaturation (EXE-DESAT), during CPET (defined as SpO2nadir≤89% and a ≥6% drop in SpO2 compared to resting levels), and b) patients without significant exertional desaturation (Non-EXED) during the CPET (SpO2nadir ≥90% and ≤5% drop).7,28 Prior to the study, patients underwent a thorough clinical examination, spirometry/lung volume assessment, carbon dioxide diffusion capacity (DLCO) measurement, and a 6MWT (performed according to recommended guidelines29). This study was approved by the Scientific Review Board Committee of Papanikolaou Hospital (804/29.05.2018), registered with ClinicalTrials.gov (NCT 03683082), and conducted in accordance with the principles of the Declaration of Helsinki. This is a secondary analysis of a larger study evaluating the effects of oxygen supplementation in IPF.25 All participants signed the informed consent form.
Experimental procedure and instrumentationPatients underwent an incremental maximal/symptom-limited CPET on a cycle ergometer (Ergoselect, McKesson, USA; work-rate increments of 10 W/min, at 50 rpm), according to guidelines.27 The protocol included a baseline/rest, a 2-min unloaded cycling, the incremental maximal test, and a 3-min recovery. A 12-lead electrocardiogram and SpO2 (Nellcore N-180, California, USA) were continuously monitored. Respiratory gas exchange was recorded by a metabolic unit (Medgraphics, Ultima CPX™, MGC Diagnostics). Cerebral oxygenation was monitored via Near-Infrared-Spectroscopy (fNIRS, Oxymon, Artinis Medical Systems Elst, The Netherlands), by measuring relative changes (μmol/l) from pre-exercise values in oxygenated (O2Hb), deoxygenated (HHb), and total (tHb) hemoglobin.30 The O2Hb and hemoglobin difference (Hbdiff) are used as indices of tissue-oxygenation, the HHb of oxygen extraction, and the tHb reflects regional changes in tissue-blood volume/local vasodilation.31 The fNIRS-sensor was placed over the left prefrontal cortex.31,32 Leg fatigue and dyspnea were assessed using the Borg CR10 (2010) scale.33
Based on previous results in cerebral-O2Hb differences during exercise in hypoxemic-COPD vs non-hypoxemic-COPD,31 we calculated (GPower software 3.1) that a sample size of 18 patients (9/group) is needed to achieve a power of 0.90 (α = 0.05, effect size=1.5). We contacted 24 patients, allowing for possible dropouts.
Statistical analysesVariables are presented as mean±SD or median (interquartile range25–75%), depending on normality of distribution. For assessing between-group differences, independent t-tests were used for normally distributed data or Mann-Whitney U test for ordinal data. NIRS data were recorded and analyzed offline (Oxysoft, Artinis Medical Systems). The average O2Hb-, HHb-, tHB-, and HBdiff-response during exercise per patient were calculated; differences between groups were compared using an independent t-test. NIRS data were also exported in 30s averages. The patient's cerebral responses at 0, 25, 50, 75, and 100% of VO2peak were determined. The effects of exercise intensity on cerebral-NIRS responses in the two groups were analyzed using two-way repeated-measures ANOVA (Group × Time), followed by Tukey Post-hoc (Statistica 7.0, StatSoft, USA). Pearson(r) or Spearman(rho) correlation was used, depending on continuous/ordinal data, to examined possible associations between variables of interest.
ResultsParticipant characteristicsTwenty-four patients with IPF without resting hypoxemia were recruited. Thirteen patients exhibiting a significant desaturation comprised the “EXE-DESAT” group, and eleven patients comprised the “Non-EXED” group. One patient terminated the test early due to a hypertensive response to exercise and was not included in the analysis. There were no significant differences between groups in anthropometric characteristics and spirometry lung volumes (Table 1); however, the EXE-DESAT group had lower (p < 0.05) diffusion capacity (%predicted DLCO).
Participant Characteristics and cardiopulmonary exercise testing (CPET) results during the incremental (ramp) protocol to exhaustion in the group with significant exertional desaturation and non-exertional desaturation.
Data are presented as means±SD or median (interquartile range 25–75%;IQ25–75); FEV1: forced expiratory volume in 1 s; FVC: forced expiratory volume; TLC: total lung capacity; DLCO: lung diffusion capacity for carbon monoxide; SpO2: Saturation from pulse oximetry; VO2, oxygen consumption; VE: minute ventilation; VE-VCO2: minute ventilation–carbon dioxide production relationship; VE-VO2: minute ventilation–oxygen uptake relationship; PETCO2: partial pressure of end-tidal carbon dioxide;
*p < 0.05 sign. vs the EXE-DESAT group.
By study design, the EXE-DESAT group exhibited greater desaturation vs Non-EXED (DSpO2: 12.2 ± 3.2% vs 3.6 ± 1.8%; p < 0.001) during CPET (Table 1). No statistically significant differences between groups were observed in V̇O2peak, peak minute ventilation (VEpeak), and ventilatory equivalent for CO2 (VE/VCO2peak), however, EXE-DESAT presented higher VE/VCO2 at ventilatory threshold (p < 0.05). At exercise termination, groups reported no significant differences in leg fatigue (p = 0.56). The EXE-DESAT reported marginally higher dyspnea vs Non-EXED (p = 0.058)
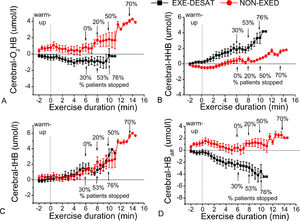
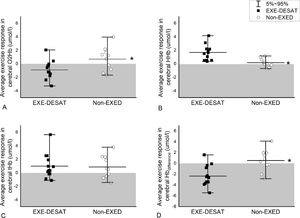
Brain oxygenationAccumulative data (all patients per group) of continuous NIRS-recordings during exercise are presented in Fig. 1. The EXE-DESAT exhibited a decline in O2Hb and a progressive rise in HHb, from the initial minutes of the CPET-exercise. The average cerebral NIRS-oxygenation response during exercise in each patient is presented in Fig. 2. The EXE-DESAT group exhibited significantly lower (p < 0.05) O2Hb and HBdiff than Non-EXED, and higher (p < 0.001) HHb, with no differences in tHb (p = 0.86).
Continuous near-infrared-spectroscopy recordings in cerebral prefrontal oxygenation. Accumulated data (mean±sd every 30 s) per group in (A) oxygenated hemoglobin (O2Hb), (B) deoxygenated hemoglobin (HHb), (C) total hemoglobin (tHB), and (D) hemoglobin difference (Hbdiff) during the maximal test in the exertional desaturation (EXE-DESAT) and non-exertional desaturation (NON-EXED) groups. The arrows depict the percentage of patients that terminated the exercise at this time (at the 6th, 8th, 10th minute). CPET: Cardiopulmonary Exercise Testing.
Average cerebral-oxygenation responses during the maximal test. Scatter interval plots depicting the average response in (A) oxygenated hemoglobin (O2Hb), (B) deoxygenated hemoglobin (HHb), (C) total hemoglobin (tHb), and (D) hemoglobin difference (Hbdifference) in patients with IPF and exertional desaturation (EXE-DESAT) and non-exertional desaturation (NON-EXED). The shaded area presents values below baseline. * p < 0.01 sign. EXE-DESAT vs NON-EXED group.
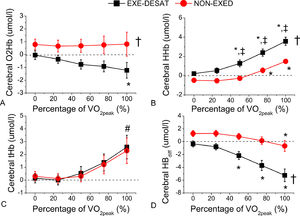
Differences between groups in cerebral-oxygenation attained at various exercise intensities (% of VO2peak) were examined (Fig. 3). Two-way ANOVAs revealed significant group differences in O2Hb, HHb, and Hbdiff, and a different pattern (p < 0.05) of increase in HHB and Hbdiff in the two groups. Specifically, EXE-DESAT presented a trend towards an HHb increase at 25% of their VO2peak (p = 0.06); then, HHb progressively increased at 50%, 75%, 100% of VO2peak (vs the beginning of loaded exercise, 0% of VO2peak; p < 0.001). In Non-EXED, significant increases in HHb were observed only at 75% and 100% (p < 0.01). In EXE-DESAT, Hbdiff significantly decreased at 50% VO2peak (p < 0.001) and continued to decrease at 75% and 100%; whereas in Non-EXED, Hbdiff significantly decreased only at 100% VO2peak (p < 0.01).
Cerebral oxygenation at different exercise intensities. (A) Oxygenated hemoglobin (O2Hb), (B) deoxygenated hemoglobin (HHb), (C) total hemoglobin (tHb), and (D) hemoglobin difference (Hbdifference) during the maximal test in patients with exertional desaturation (EXE-DESAT) and non-exertional desaturation (NON-EXED). †p < 0.001 sign. EXE-DESAT vs NON-EXED group; *p < 0.05 sign. vs the beginning of the test (0%) within the same group; ‡p < 0.05 sign. vs respective intensity in EXE-DESAT; #p < 0.05 sign. vs 25, 50, and 75% of VO2peak within both groups; VO2peak: peak oxygen uptake.
Cerebral-NIRS parameters during CPET were significantly correlated with lung diffusion capacity, 6MWT, and dyspnea (Appendix A-Fig.1S-4S). In detail, the average cerebral-O2HB response was correlated (Fig. 1S) with dyspnea (rho = ‒0.71, p < 0.05), CPET-duration (r = 0.42, p < 0.05), %predicted DLCO (r = 0.53, p < 0.01), and 6MWT-distance (r = 0.58, p < 0.01). The average-HHb (Fig. 2S) was correlated with dyspnea (rho=0.57, p < 0.05), %predicted DLCO (r = -0.43, p < 0.05), and 6MWT-distance (r = ‒0.50, p < 0.05). The average-HBdiff (Fig. 3S) was correlated with dyspnea (rho=‒0.66, p < 0.05), %predicted DLCO (r = 0.57, p < 0.01), 6MWT-distance (r = 0.60, p < 0.01), and 6MWT-desaturation (r = -0.65, p < 0.001). Furthermore, the O2Hb values obtained at 25% VO2peak, were significantly correlated (Fig. 4S) with CPET-duration (r = 0.49, p < 0.05) and CPET-dyspnea (rho = ‒0.75, p < 0.05).
DiscussionOur primary outcomes were that patients with IPF and isolated exertional desaturation presented significantly lower cerebral-oxygenation and a differential pattern in this response during CPET-exercise compared with patients without exertional desaturation. That is, patients with exertional desaturation exhibited an inability to maintain cerebral-oxygenation from low exercise intensities (below 50% VO2peak). In contrast, patients without exertional desaturation exhibited significant deoxygenation at high exercise intensity (above 75% of VO2peak). Secondary outcomes were that cerebral-oxygenation/deoxygenation indices were significantly correlated with CPET-exercise duration and dyspnea, diffusion capacity, and 6MWT.
In healthy adults, the increase in O2Hb and Hbdiff, coupled with a decrease in HHb during exercise has been suggested to reflect an enhanced cortical neuronal activity.17–19 In contrast, O2Hb reductions and significant HHb elevations have been described in cerebral ischemia.34 Thus, in our study, the early decrease in cerebral-O2HB and Hbdiff below baseline levels and the large increases in HHb from low exercise intensity reflect significant impairments in cerebral-oxygenation, as also evidenced in patients with COPD by Higashimoto et al.31 In contrast, in patients without exertional desaturation, cerebral-Hbdiff was significantly reduced only at high exercise intensity (around 75% VO2peak), above the critical power,8 mimicking the pattern described in healthy individuals.17–19,35,36 However, it should be noticed that despite the higher overall cerebral-O2Hb and HbDiff responses in the non-desaturators group, the increase in O2HB during exercise was still blunted, as indicated by the slightly higher than baseline values. This finding suggests limitations in cerebral-oxygenation during exercise, even without significant exertional desaturation in patients with IPF. The similar tHb-responses between groups suggest similar vasodilation/blood volume changes in the cerebral-microvasculature during exercise.31
An interesting finding in this study is the significant impact of cerebral-oxygenation on exercise duration and dyspnea. Patients that exhibited reduced cerebral-O2Hb from low exercise intensity (25% of VO2peak), had shorter exercise duration and presented greater dyspnea. Dyspnea, a subjective experience of breathing discomfort,13 was marginally higher at exercise termination in EXE-DESAT compared with the Non-EXED group, even though VO2peak did not differ between groups. In hypoxemic patients with COPD, exertional dyspnea was correlated with impaired cerebral-oxygenation and HHb-elevations during exercise, but not SpO2 during CPET.23,31 In our study, cerebral-NIRS variables were more strongly correlated with dyspnea than with the magnitude of desaturation during CPET. Although cerebral-deoxygenation was not significantly correlated with leg fatigue ratings, this does not suggest that skeletal muscles are not involved in exercise intolerance in patients with IPF. In fact, our patients rated higher their leg fatigue than dyspnea, which is expected during cycling, as previously discussed,8 highlighting the importance of skeletal muscles in exercise limitations in these patients.
Importantly, the significant correlation between brain-oxygenation indices and DLCO, suggests an association between cerebral-deoxygenation and diffusion limitations. Lung diffusion reduction is a known predictor of exercise-induced hypoxemia and disease outcomes/severity.4,37 Both groups presented ventilatory inefficiency during exercise (as evidenced by high peak-VE/VCO2 and VE/VCO2 at ventilatory-threshold), however, the higher VE/VCO2 at ventilatory threshold in the exertional-desaturators group, is suggestive of greater ventilation/perfusion mismatch in this group.9,11 Groups did not exhibit signs of significant pulmonary hypertension during CPET, as peak oxygen-pulse (VO2/HR) was >95% of predicted and VE/VCO2 at ventilatory threshold was <45.11,38
This study has both strengths and limitations. Τwo strict criteria for defining exertional desaturation (SpO2<88% and ≥6% drop in SpO2) were used, as the cycling test is less sensitive to diagnose desaturation than the 6MWT.8 This allowed for a substantial difference in desaturation between groups. Groups with similar resting-SpO2 were used because an exertional nadir of an absolute value of SpO2≤89% would have resulted in a different magnitude of SpO2 decline in patients with various resting saturation levels.39 Cerebral-oxygenation was assessed with NIRS, a noninvasive, reliable technique for measuring brain function/oxygenation, during exercise.20,30 Cerebral-NIRS measurements were obtained from the left pre-frontal cortex, as activation in this area has been associated with exertional dyspnea in COPD.23,40 Other brain areas (premotor/motor regions) important in dyspnea perception,17 were not examined. However, studies using multichannel-NIRS in COPD, showed that the pattern of cortical-deoxygenation during exercise was similar in prefrontal, premotor, and motor regions.17 Finally, all participants were on anti-fibrotic medication; thus, results cannot be extrapolated to patients not using IPF-medication. Although ATS/ERS Guidelines on IPF treatment listed antifibrotic treatment as "conditional", most IPF-expert pulmonologists agree that antifibrotic treatment should be started as soon as the disease is diagnosed.2,41
Clinical applicationOur data highlight the importance of selecting the appropriate exercise intensity in patients with IPF and exertional desaturation to maintain adequate brain oxygenation during exercise. Modifications in exercise intensity may be considered in IPF patients with exertional desaturation and low DLCO (<50%), as brain ischemia might develop when exercising even at moderate intensity i.e. at 50–60% of VO2peak which is often used in COPD patients. Alternatively, if higher exercise intensity is desired, oxygen supplementation25 or intermittent exercise,42 may be considered. However, the impact of different exercise modalities on cerebral-oxygenation requires further studies. Longer duration of exercise rehabilitation programs will allow slower progression of training. Beyond planned exercise, mild daily life physical activity might also cause cerebral hypoxia in patients with IPF. Brain oxygenation measurements could assist in identifying patients with significant exertional cerebral-deoxygenation.
ConclusionsPatients with IPF and isolated exertional desaturation exhibit significantly lower cerebral-oxygenation during incremental exercise than patients without exertional hypoxemia. Importantly, in patients with exertional desaturation inadequate cerebral-oxygenation develops at low/moderate exercise intensity (below 50% of VO2peak), whereas in patients without exertional desaturation significant impairments in cerebral-oxygenation develop at high-intensity exercise. The significant associations of impaired cerebral-oxygenation with shorter exercise duration, higher dyspnea, and lower DLCO, suggest a more severely compromised cerebral-oxygenation during exercise in patients with greater diffusion limitations. Our findings support the implementation of longer-duration rehabilitation programs so that lower intensity can be applied at the initial stages in these patients with IPF and highlight the need for personalized, IPF-specific exercise programs.
AuthorshipDipla K, Boutou AK, Zafeiridis A, and Markopoulou A conceived this study and supervised its implementation. Pitsiou G, Stanopoulos I, Kioumis I collaborated in the inception of the study and interpretation of data. Dipla K, Boutou AK, Zafeiridis A, Papadopoulos S, and Kritikou S collected the data and collaborated in the analysis. All the authors contributed to the interpretation of the results and revisions and approved the manuscript.