Human telomerase reverse transcriptase (hTERT) is the catalytic subunit of telomerase enzyme, which adds nucleotides to telomeres and counteracts their length shortening. The development of a telomere maintenance mechanism represents a hallmark of cancer. On the other hand, idiopathic pulmonary fibrosis (IPF) is associated with mutations in telomerase genes and shorter telomeres. IPF is frequently complicated with lung cancer.
AimTo investigate the expression of hTERT in lung cancer with co-existing IPF and to compare with lung cancer without fibrosis.
MethodsDiagnostic lung cancerous biopsies were retrieved from 18 patients with lung cancer and concomitant IPF, as well as 18 age and gender matched controls with lung cancer without pulmonary fibrosis. The expression of hTERT was studied with immunohistochemistry. ImajeJ software was used to quantitate subcellular stain intensity. Immunohistochemical investigation of two senescence-associated markers, p16 and p21, was also performed in all 36 cases.
ResultsBoth groups highly expressed hTERT, without significant difference (100% vs 95%, p = 0.521). Evaluation of p16 and p21 immunostaining revealed negative to minimal immunoreactivity in both groups. hTERT localization exhibited higher median nuclear intensity in the group of lung cancer with IPF (0.62 vs 0.45, p = 0.016), while cytoplasmic intensity did not differ significantly (0.17 vs 0.15, p = 0.463). Higher median nuclear intensity was also correlated with small cell lung cancer subtype in the whole study sample (0.69 vs 0.45, p = 0.09).
ConclusionhTERT is highly expressed in lung cancer with concomitant IPF, but with differential localization compared to lung cancer without IPF, implying differences in pathogenicity and requiring further investigation.
Telomeres are tandem repeats of TTAGGGs sequences protecting the ends of the linear chromosomes and acting as the “biological clock” of cells; their length is gradually shortened after each cell division, due to incomplete lagging-strand DNA synthesis, and when they reach a critical length they introduce the cells into a permanent growth arrest signaling process (replicative senescence).1 Telomerase is an enzyme that adds nucleotides at the ends of the chromosomes and prevents their shortening. Its main components are the catalytic subunit, human telomerase reverse transcriptase (hTERT), and the human telomerase RNA component (hTERC), which serves as a template for telomere replication.1,2 In humans, telomerase is normally expressed during embryonic development, and later silenced in most somatic cells on differentiation.2
Importantly, the activation of a telomere maintenance mechanism represents a hallmark of cancer,3 enabling the cancer cells to avert senescence and divide limitlessly. The maintenance of telomeres is achieved either by reactivation of telomerase, which occurs in 85-90% of all human cancers, or by activating telomerase-independent, recombination pathways, known as the alternative lengthening of telomeres (ALT) pathway, occurring in 10-15% of cancers.4,5 The reactivation of telomerase mainly involves the overexpression of hTERT, which is the rate-limiting component of telomerase.4,6 Regarding the ALT pathway, it relies on homologous recombination in order to lengthen telomeres, it is more frequent in tumors of mesenchymal origin and associated with unfavorable prognosis.7
On the other hand, mutations in components of telomerase may result in shorter telomeres and phenotypes of premature ageing syndromes which have been termed telomeropathies,8 indicating that tissues must maintain an optimum level of telomerase in order to support homeostasis.2 The most well-characterized telomeropathies include aplastic anaemia, dyskeratosis congenita and familial idiopathic pulmonary fibrosis (IPF).1 It has been demonstrated that not only familial but also sporadic IPF, as well as other interstitial lung diseases (ILDs) are associated with mutations in hTERT gene or other components of telomerase, with single nucleotide polymorphisms in telomere-related genes, and/or with shorter telomeres.9-13
Interestingly, IPF is an independent risk factor of lung cancer (LC) development with an approximately 5-fold relative risk for patients with IPF to develop lung cancer, compared to general population.14-16 Lung tumors in patients with IPF, contrary to general population, are predominately peripheral squamous carcinomas in proximity to fibrotic lesions, and less frequently adenocarcinomas, which may exhibit rare subtypes.17,18 Evidence suggests a possible pathogenic link between the two diseases, involving genetic and epigenetic alterations and aberrant interplay between the activated stroma cells and the dysplastic epithelium.19,20 The hyperproliferative lesions of bronchiolar epithelium, lying within honeycomb cysts have been suggested as preneoplastic lesions.20,21
In the present study we investigated the immunohistochemical expression of hTERT in lung cancer tissues with or without concomitant IPF. We hypothesized that the expression might differ between the two groups due to possible differential expression patterns of telomerase in the precursor lesions of each group. Since telomerase activation in cancer is correlated with evasion to senescence, we additionally investigated the immunohistochemical expression of p16INK4A and p21WAF1/CIP1, two cyclin-related kinase inhibitors that are parts of p16INK4A/Retinoblastoma (Rb) and p53/ p21WAF1/CIP1 tumor suppressor pathways,22 and represent well-established markers indicative of senescence.23
MethodsPatientsPatients’ records were retrieved from a previously published IPF-lung cancer registry from our unit, which was a part of a multi-center national epidemiological study.14 In that registry, IPF was diagnosed according to the most recent international guidelines, and lung cancer was confirmed with pathologic or cytologic diagnosis. For the purpose of the present study, we identified the cases with (a) pathologic diagnosis (b) diagnostic biopsy from lung tissue (c) adequate archival tissue. Finally, 18 patients enrolled in the study (IPF-LC group).
For each patient, a sex and age matched control with lung cancer, without pulmonary fibrosis was assigned (LC group). Patients were identified from the Oncology Unit of our Hospital, and the absence of pulmonary fibrosis was confirmed after review of baseline chest Computed Tomography scans from two pulmonologists with expertise in ILD (VT, DB). The study was conducted in “Sotiria” General Hospital for Diseases of the Chest, Athens, Greece. The study protocol was approved from the Institutional Review Board of our Hospital (IRB approval number 24811/12-12-2017). All patients provided written consent.
Tissue preparation and immunohistochemistryTissue deparrafinization and epitope retrieval were performed with the use of Dako-Agilent PT Link Instrument and Envision FLEX Target Retrieval Solution. The immunohistochemical staining was made automatically with Autostainer Link 48 (Dako-Agilent). The antibody TERT (mouse monoclonal antibody, clone 2D8, Invitrogen) was used at a dilution of 1/100 and 30 minutes incubation at room temperature. Additionally, the antibodies p16 (mouse monoclonal antibody, clone BC42, Biocare) and p21 (mouse monoclonal antibody, clone DCS-60.2, Zeta Corporation) were used at a dilution of 1/75 and 1/100 respectively and 30 minutes incubation at room temperature. DAB (3,3′-Diaminobenzidine) was used as chromogen.
Immunohistochemical evaluationThe slides were blinded and interpreted by two pathologists (CM, IV). A minimum of 5 random high power fields were selected for evaluation. Εach 100 cancer cells from cancerous tissues were assessed to determine the positive-staining rate. Although TERT is basically a nuclear stain, cytoplasmic expression was also considered as positive, as TERT is expressed in both nucleus and cytoplasm in cancer cells.24 Moreover, in order to objectively evaluate the stain intensity, ImajeJ software25 was used. The staining intensity was quantitatively evaluated considering the mean grey value of color deconvoluted images.26 Microphotographs at x200 magnification of the stained slides were taken, the DAB stain was then separated by color deconvolution and converted into grayscale using the Qupath 0.2.0 software,27 and the grayscale images were exported to ImageJ software, where the mean gray value of the epithelial cells in question (tumoral cells) and the bystanding lymphocytes was measured. In order to normalize for staining intensity variation across samples due to preanalytical factors, the epithelial/lymphocyte mean gray value ratio was calculated for each slide. This ratio was multiplied by the percentage of positive cells for each case. Regarding p16 and p21 expression, nuclear staining was considered positive.
Statistical analysisQuantitative variables were expressed as mean (Standard Deviation) or as median (interquartile range). Qualitative variables were expressed as absolute and relative frequencies. Students’ t-tests or Mann-Whitney test were used for the comparison of continuous variables between two groups. For the comparison of proportions chi-square and Fisher's exact tests were used. Spearman correlations coefficients were used to explore the association of two continuous variables. All reported p values are two-tailed. Statistical significance was set at p<0.05 and analyses were conducted using SPSS statistical software (version 23.0).
ResultsThe sample consisted of 36 males with lung cancer with mean age 71.1 years (SD=7.9 years), half of whom were also diagnosed with IPF. Patients’ characteristics are presented in Table 1. Age and smoking history (pack-years) were similar in both groups (p > .05). The subtype of cancer was similar in both groups (p > .05), with 77.8% of the patients in each group having Non-Small Cell Lung Cancer (NSCLC) subtype. Among patients with IPF-LC, 35.3% were under antifibrotics (pirfenidone or nintedanib). All patients with IPF were sporadic cases; there were no patients with familial IPF.
Baseline patients’ characteristics.
| Number of patients | IPF-LC 18 | LC 18 | p | |
|---|---|---|---|---|
| Age (years), mean±SD | 72.8 (7.2) | 69.3 (8.3) | 0.181+ | |
| PY, mean (SD) | 49.3 (32.4) | 48.5 (34.2) | 0.946+ | |
| NSCLC, N(%) | ||||
| No | 4 (22.2) | 4 (22.2) | 1.000‡‡ | |
| Yes | 14 (77.8) | 14 (77.8) | ||
| Adenoca, N(%) | ||||
| No | 12 (66.7) | 12 (66.7) | 1.000‡ | |
| Yes | 61 (33.3) | 6 (33.3) | ||
| Squamous,N(%) | ||||
| No | 9 (50.0) | 10 (55.6) | 0.738‡ | |
| Yes | 91 (50.0) | 8 (44.4) | ||
| SCLC, N(%) | ||||
| No | 14 (77.8) | 14 (77.8) | 1.000‡‡ | |
| Yes | 4 (22.2) | 4 (22.2) | ||
| Antifibrotics,N (%) | ||||
| No | 11 (64.7) | NA | ||
| Yes | 6 (35.3) | NA | ||
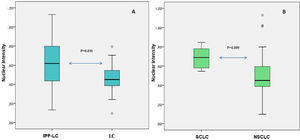
The median percentage of hTERT positive cells did not differ significantly between the two groups; it was 1.00 (100%) (IQR 0.90 – 1.00) for patients with IPF and 0.95 (95%) (IQR 0.80 – 1.00) for patients without IPF, p > 0.05 (Table 2). All cases showed both nuclear and cytoplasmic expression of the protein and the median nuclear intensity was greater than cytoplasmic in both groups. When comparing the stain intensity between the groups, nuclear intensity was significantly greater in patients with IPF, with median being 0.62 (0.44 0.80) in those with IPF and 0.45 (0.39 0.54) in those without IPF, p = 0.016 (Fig. 1A). On the contrary, cytoplasmic intensity was similar in both groups. More specifically, the median cytoplasmic intensity was 0.17 (0.11 0.35) for patients with IPF and 0.15 (0.09 0.21) for patients without IPF (p > 0.05). Characteristic tissue images are shown in Fig. 2.
Percentage of hTERT positive cells, Nuclear, and Cytoplasmic Intensity of the two studied groups and association with patients’ characteristics.
| Percentage of hTERT positive cells | p | Nuclear intensity | p | Cytoplasmic intensity | p | |
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | ||||
| IPF-LC | 1.00 (0.90 – 1.00) | 0.521++ | 0.62 (0.44 0.80) | 0.016++ | 0.17 (0.11 0.35) | 0.463++ |
| LC | 0.95 (0.80 1.00) | 0.45 (0.39 0.54) | 0.15 (0.09 0.21) | |||
| Age r+ | 0.559 | 0.280 | 0.288 | |||
| PY r+ | 0.589 | 0.810 | 0.527 | |||
| NSCLC | ||||||
| No | 1 (0.93 1) | 0.438++ | 0.69 (0.58 0.78) | 0.009++ | 0.10 (0.09 0.21) | 0.216++ |
| Yes | 1 (0.8 1) | 0.45 (0.39 0.59) | 0.17 (0.13 0.34) | |||
| Adenoca | ||||||
| No | 0.98 (0.75 1) | 0.327++ | 0.55 (0.39 0.69) | 0.920++ | 0.14 (0.09 0.24) | 0.044++ |
| Yes | 1 (0.9 1) | 0.52 (0.43 0.64) | 0.27 (0.14 0.46) | |||
| Squamous | ||||||
| No | 1 (0.9 1) | 0.196++ | 0.57 (0.45 0.77) | 0.041++ | 0.16 (0.10 0.45) | 0.575++ |
| Yes | 0.9 (0.7 1) | 0.43 (0.31 0.58) | 0.15 (0.13 0.25) | |||
| SCLC | ||||||
| No | 1 (0.8 1) | 0.438++ | 0.45 (0.39 0.59) | 0.009++ | 0.17 (0.13 0.34) | 0.216++ |
| Yes | 1 (0.93 1) | 0.69 (0.58 0.78) | 0.10 (0.09 0.21) | |||
| Antifibrotics1 | ||||||
| No | 1 (0.8 1) | 0.410++ | 0.64 (0.44 0.80) | 0.920++ | 0.17 (0.10 0.35) | 0.777++ |
| Yes | 1 (1 1) | 0.62 (0.43 0.85) | 0.25 | (0.24 0.35) |
Abbreviations: TERT: Telomerase Reverse Transcriptase; SD: Standard Deviation; IQR: Interquartile range; PY: Pack-Years; NSCLC: Non-small cell lung cancer; Adenoca: adenocarcinoma; SCLC: small cell lung cancer.
Differential nuclear intensity between groups. Fig. 1A. Box plots for nuclear intensity of hTERT immunostaining in the group of IPF-LC and in the group LC. Fig. 1B. Box plots for nuclear intensity in the cases of SCLC and in the cases of NSCLC. Nuclear intensity is quantified as the mean grey value of color deconvoluted images as per algorithm of the software used.
Abbreviations: IPF: Idiopathic Pulmonary Fibrosis; LC: Lung Cancer; SCLC: Small Cell Lung Cancer; NSCLC: Non-Small Cell Lung Cancer; hTERT: human telomerase reverse transcriptase.
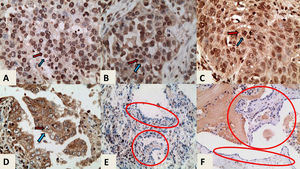
hTERT immunostaining, ×400 magnification. In each case the blue arrow indicates the nucleus and the red arrow indicates the cytoplasm A. High nuclear and very low cytoplasmic expression in a case of LC without fibrosis. B. High nuclear and moderate cytoplasmic expression in a case of LC without fibrosis C. High nuclear and moderate cytoplasmic expression in a case of IPF-LC. D. Low nuclear and high cytoplasmic expression in case of LC without fibrosis. E and F. hTERT immunostaining, x200 magnification. Healthy adjacent tissues of cases C and D with negative expression of hTERT in pneumonocytes (indicated in circled areas).
Abbreviations: IPF: Idiopathic Pulmonary Fibrosis; LC: Lung Cancer; hTERT: human telomerase reverse transcriptase.
The percentage of hTERT positive cells was not significantly associated with any of patients’ characteristics (Table 2). Nuclear intensity was significantly greater in patients with SCLC subtype and significantly lower in patients with NSCLC (p = 0.009) or squamous subtype (p = 0.041) (Fig. 1B). Cytoplasmic intensity was significantly greater in patients with adenocarcinoma subtype (p = 0.044).
Evaluation of senescence-associated markers p16 and p21 demonstrated that all 36 samples were negative for p16 and the majority of cases (31/36) were minimally immunoreactive to p21, except 5 cases which did not show any immunoreactivity. More specifically, in the majority of samples the expression of p21 was nuclear, with weak stain intensity, and the percentage of positive cancer cells varied from 1-10%. Characteristics images of p16 and p21 immunostaining are provided as Supplement Figures.
DiscussionIn the present study, we examined the hypothesis that the immunohistochemical expression of hTERT may differ in tumor tissues of patients with lung cancer with or without co-existing IPF. We found that hTERT is highly expressed (>85% positive cells) in both groups without significant difference, but interestingly, nuclear intensity was significantly greater in patients with IPF. There was no association between the percentage of hTERT-positive cells and the clinicopathological characteristics of the cases. Moreover, greater nuclear intensity was reported in SCLC compared to NSCLC cases. Further immunohistochemical analysis with senescence-associated markers p16 and p21 revealed that no sample was immunoreactive to p16 and the majority of samples were minimally immunoreactive to p21 antibody.
Very few studies exist reporting the degree of hTERT subcellular intensity in tumor tissues and its correlation with clinicopathological characteristics. A recent study investigated subcellular expression of hTERT in cervical cancer with co-existing human papillomavirus (HPV) infection; higher nuclear expression of hTERT was associated with certain species of HPV.28 In another study, higher nuclear intensity of hTERT was found in metastatic lymph node biopsies compared to primary tumors of nasopharyngeal carcinomas, suggesting a possible involvement to metastatic potential.29 Differential subcellular expression of hTERT among different lung cancer types has previously been demonstrated reporting that SCLC presents diffuse nuclear expression in contrast to restricted nucleolar localization observed in NSCLC.30 Although we did not observe a nucleolar expression pattern in NSCLC in our cohort, we detected more pronounced nuclear staining in SCLC cases, which may correlate with the fast-growing nature of such tumors and the implication of anti-apoptotic pathways in SCLC pathogenesis, which have been associated with nuclear retaining or hTERT.31,32 Although the evidence of our study is descriptive and insufficient to address an explanation, it indicates the differential pathogenicity and origin between the groups, which is profound in the case of SCLC and NSCLC but also putative between IPF-LC and LC. Of note, all patients in our IPF-LC cohort were sporadic cases of IPF, and still, data implies potential differences in pathogenicity of lung cancer development and telomere-related biological mechanisms.
It is well known that telomerase activation is the predominant telomere maintenance mechanism of cancer cells.33 The most frequent alterations leading to telomerase activation are hTERT promoter mutations, hTERT gene rearrangements and DNA copy amplifications, and epigenetic alterations.34 Approximately, 4–11% of tumors use ALT pathway, a homologous recombination-based pathway which is prevalent in cancers from the mesenchymal origin and usually associates with poor clinical outcome.7 IPF and other ILDs are associated with mutations in telomerase genes and with shorter telomeres.9,12 In the present study, we demonstrated that even in the case of background pulmonary fibrosis, hTERT protein is highly expressed in cancerous lung tissues. Similarly, to IPF, cirrhosis, another chronic fibrotic disease, is correlated with shorter telomeres and it is well-established that it predisposes to the development of hepatocellular carcinoma (HCC). Notably, HCC with background cirrhosis demonstrates high levels of telomerase activity, mainly due to hTERT promoter mutations.35 Interestingly, it has been suggested that this genetic alterations represent an early step during hepatocarcinogenesis on cirrhotic livers, possibly due to its necessity in order to escape senescence, contrary to the development of HCC on hepatocellular adenomas, when hTERT promoter mutations occur later, after acquisition of CTNNB1 (Catenin Beta 1) driver mutations.35,36
Preclinical research in lung cancer cell lines has highlighted the role of p53/ p21WAF1/CIP1 pathway in regulation of hTERT expression since it has been demonstrated that hTERT is downregulated via p53 pathway upon treatment with telomerase inhibitors.37 Impaired telomere function is associated with induction of replicative senescence and cell cycle arrest; in contrast, reactivation of telomerase is considered to enable cancer cells to escape senescence.1 The immunohistochemical investigation of our sample with two senescence-associated markers, the cell cycle inhibitors p16 and p21, which are involved in major tumor suppressor pathways p16INK4A/Rb and p53/ p21WAF1/CIP1, revealed negative to minimal immunoreactivity in both groups. The absence of senescence-associated markers is consistent with the overexpression of hTERT in both groups and indicates evasion to senescence and sustained proliferation.
Intriguingly, the regulation of the subcellular localization of hTERT has not been fully clarified yet. Besides its usual nuclear localization, hTERT is found in the cytosol and mitochondria and it contains two sequences that regulate its transport in and out of organelles: a nuclear targeting signal sequence, and a mitochondrial targeting sequence.34 It has been described that oxidative stress leads to translocation of hTERT from the nucleus into the cytosol and mitochondria, but different cells demonstrate heterogeneity of telomerase export upon stress induction.38,39 It has been suggested that mutations in the nuclear export signal (NES) of hTERT render the protein unable to shuttle from the nucleus, which was correlated to an increase in nuclear DNA damage.40 Mechanistic studies have demonstrated that the translocation of hTERT from the nucleus depends on tyrosine-phosphorylation by the Src kinase family and further investigation revealed that tyrosine phosphatase Shp-2 protein is a negative regulator of tyrosine 707 phosphorylation and leads to inhibition of hTERT nuclear export.41 The nuclear retaining of hTERT might be associated with further effects; interestingly, there is published data of a protective role of nuclear hTERT against apoptosis.31,38 Also, hTERT is considered to be involved in non-canonical, extra-telomeric functions, not only when located in the cytoplasm, as it was initially reported, but also in the nucleus; those functions include non-telomeric DNA damage responses, modulation of chromatin, acceleration of cell cycle kinetics, and control of mitochondrial integrity following oxidative stress.42
Lung cancer is a frequent complication in patients with IPF. The pathogenic mechanisms occurring in IPF recall those involved in carcinogenesis, which could be therapeutically exploited for each disease entity as well as for this disease combination.20 Indeed, the management of patients with IPF and LC is quite challenging, since the majority of therapeutic interventions may trigger an acute exacerbation of pulmonary fibrosis.43,44 Novel strategies to target this disease combination are sorely needed.43 To this end, deciphering the specific mechanisms of lung tumorigenesis in the context of pulmonary fibrosis, including the telomere maintenance mechanism, could broaden therapeutic avenues for both diseases.20,45
The present study has several limitations. Firstly, it is a unicentric study with small sample size. Secondly, even though the controls were sex- and age-matched, and with matched smoking history, nevertheless, the case-control design of the study might have let the interference of other confounding factors. Thirdly, the immunohistochemical expression of hTERT does not strictly reflect telomerase activity; further studies with other PCR-based assays might be needed. Also, in the present study we provide descriptive findings of hTERT localization, which require further investigation with molecular and mechanistic studies. Finally, further research on fibrotic, non-cancerous, lung tissues and, ideally, a similar investigation in a well-characterized population of patients with IPF-LC and known mutations in telomere-related genes, are essential to investigate the role of telomerase reactivation during carcinogenesis in the context of pulmonary fibrosis.
ConclusionIn conclusion, we provide evidence that hTERT is highly expressed in lung cancer tissues of patients with concomitant IPF, without significant difference compared to patients with lung cancer without pulmonary fibrosis. Nuclear localization of the stain was significantly higher in patients with IPF and lung cancer and it was positively correlated with SCLC subtype. Further research is needed to validate the results and provide molecular and mechanistic evidence of lung carcinogenesis in the context of pulmonary fibrosis.
FundingThis research is co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme «Human Resources Development, Education and Lifelong Learning 2014-2020» in the context of the project “The role of telomeres and telomerase in IPF-associated lung cancer” (MIS 5047957).