It is becoming increasingly evident that neutralizing antibodies as well as T-cell-mediated immune responses (acquired through natural SARS-CoV-2 infection or vaccination against the SARS-CoV-2 spike protein) are crucial to protect against COVID-19.1-5 Evidence suggests that T-cell responses are required for durable immunity against COVID-19, including emerging SARS-CoV-2 variants of concern (VOC), and are implicated in reducing disease severity.6 While VOC may partially escape the humoral response, functional preservation of vaccine-induced T-cells allows VOC recognition independent of timing or regimen.3,7 Therefore, monitoring T-cell responses may provide a more comprehensive picture of COVID-19 immunity; however, data are limited on the magnitude and durability of T-cell immunity following two doses versus booster dose responses.
We previously carried out a feasibility study using the QIAreach™ Anti-SARS-CoV-2 Total Test (QIAGEN, Hilden, Germany) and QuantiFERON SARS-CoV-2 Research Use Only (QFN SARS-CoV-2) assay (QIAGEN, Germantown, USA) to measure durability of total antibody and T-cell-mediated responses, respectively, in 12 subjects during and after the 2-dose mRNA vaccination regimen (mRNA-1273 [Moderna]) and in 4 PCR-confirmed COVID-19 convalescent subjects.8 We showed that vaccinated individuals had robust antibody and CD4+/CD8+ T-cell responses to a SARS-CoV-2 mRNA vaccine for 2 months following completion of the initial 2-dose regimen.8 However, in most individuals, T-cell response declined between first and second doses, demonstrating the need for a second dose.8
Here, we report on the QFN SARS-CoV-2 testing in an expanded cohort of COVID-19-naïve, healthy volunteers to measure the durability of mRNA vaccine T-cell responses for up to 40 weeks following the initial 2-dose regimen (mRNA-1273 [Moderna] or BNT162b2 [Pfizer-BioNTech] vaccines), pre-booster, and post-booster (matched to their initial regimen; received at median 8.6 [range: 7.2–9.0] months after completing the initial vaccination regimen). To measure QFN SARS-CoV-2 T-cell response, whole blood specimens were collected from: 26 subjects at days 14–20 (2 weeks) following completion of the initial 2-dose vaccination regimen (mRNA-1273, n=13; BNT162b2, n=13); 24 subjects at days 182–252 (7–9 months; pre-booster dose) (mRNA-1273, n=13; BNT162b2, n=11); and 7 subjects up to 3 months post-booster. Details on plasma extraction were described previously.1 The QFN SARS-CoV-2 assay consists of three antigen (Ag) tubes, SARS-CoV-2 Ag1, Ag2, and Ag3. The Ag1 tube contains CD4+ epitopes derived from the S1 subunit (receptor-binding domain) of the spike protein, Ag2 contains CD4+ and CD8+ epitopes from the S1 and S2 subunits of the spike protein, and Ag3 comprises CD4+ and CD8+ epitopes from S1 and S2, plus immunodominant CD8+ epitopes derived from the whole genome.8 Reactive T-cell response was defined as an interferon-gamma (IFN-γ) value of ≥0.15 international units (IU)/mL greater than the background value from the QFN SARS-CoV-2 Nil tube.3 Each QFN SARS-CoV-2 Ag Nil subtracted unpaired response was compared between vaccine types by Mann-Whitney test (2 weeks versus 7–9 months after the initial 2-dose regimen). Wilcoxon matched-pairs signed rank test was used to compare pre-booster and 3-month post-booster responses. Loss to follow-up was minor (n=9 at days 182–252 testing, comprising n=5/13 for mRNA-1273 and n=4/11 for BNT162b2); and there were no notable differences in clinical and demographic characteristics between those lost to follow-up versus other study participants at days 182–252.
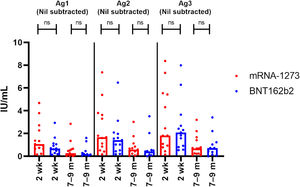
We found no statistically significant differences in T-cell responses between subjects receiving the mRNA-1273 and the BNT162b2 vaccines at any timepoint (Fig. 1). Following completion of the initial 2-dose mRNA-1273 regimen, median Ag1, Ag2, and Ag3 IFN-γ values (Nil subtracted) were 1.1, 1.7, and 1.8 IU/mL, respectively; all subjects were classified as reactive. Upon completion of the durability study (median follow-up for mRNA-1273 regimen: 8.5 [range: 6.5–9.1] months), these values (Nil subtracted) were 0.27, 0.59, and 0.69 IU/mL, respectively; 11/13 (84.6%) subjects were reactive. Upon completion of the 2-dose BNT162b2 regimen, median Ag1, Ag2, and Ag3 IFN-γ values (Nil subtracted) were 0.68, 1.4, and 2.1 IU/mL, respectively; all subjects were reactive. After the median 7.2 [range: 6.6–9.4] months’ follow-up for the BNT162b2 regimen, these values (Nil subtracted) were 0.18, 0.42, and 0.72 IU/mL, respectively; 8/11 (72.7%) subjects were reactive.
Comparison of QFN SARS-CoV-2 antigen tube (Nil subtracted) T-cell mediated immune response in subjects receiving an initial 2-dose vaccination with the mRNA-1273 or BNT162b2 vaccines. A Mann-Whitney test was used to compare responses between vaccines at the 2-week and 7–9-month timepoints.
Dots represent individual test points and columns represent median responses within the test cohort. Not all subjects had specimens collected at all timepoints.
Ag, antigen tube; IU, international units; m: months; ns: non-significant; wk, weeks.
Compared with pre-booster levels, elevated Ag1, Ag2, and Ag3 responses were observed within 7 days after receiving a booster dose (mRNA-1273, n=6; BNT162b2, n=1), with IFN-γ levels increasing at 1 month post-booster but declining by 3 months (Fig. 2). At 3 months post-booster, there were no statistically significant differences between pre- and post-booster Ag1, Ag2, and Ag3 IFN-γ levels, regardless of vaccine.
Comparison of QFN SARS-CoV-2 antigen tube (Nil subtracted) T-cell mediated immune response in subjects pre- and post-booster vaccination (whole blood specimens collected at 0 months [≤7 days], 1 month [18–32 days], and 3 months [81–96 days] following the booster). Wilcoxon matched-pairs signed rank test was used to compare pre-booster and 3-month post-booster responses.
Dots represent individual test points and columns represent median responses within the test cohort. Not all subjects had specimens collected at all timepoints.
Ag, antigen tube; IU, international units; m: months; ns: non-significant.
In this longitudinal study, T-cell mediated immune responses were sustained for 9 months following the 2-dose mRNA vaccination regimen in COVID-19-naïve individuals, with no significant differences between vaccines. In response to a booster dose, T-cell responses initially increased before stabilizing to pre-booster levels 3 months post-booster.
This is one of the few longitudinal studies exploring the durability of cellular immunity generated in response to mRNA vaccines and providing insights into vaccine efficacy; even fewer longitudinal studies have investigated efficacy against VOC. A spike in antibody responses has been reported after booster vaccination but the durability of these responses is yet to be determined;3 however, T-cell responses are generally maintained at pre-booster levels and potentially provide longer-term protection,3 especially against severe disease as protective T-cell immunity may correlate with milder clinical manifestations.1,3,5 Notably, this has implications for immunocompromised individuals who may be at risk of severe COVID-19 but have attenuated humoral responses to vaccination.9 Gaining a better understanding of the cellular immune response to COVID-19 should be an important point of future research, in order to inform public health policies and guide targeted interventions for vulnerable populations.6 Further research is also needed to investigate whether immune response monitoring can identify correlates of disease protection5 or has a role in COVID-19 clinical management.
FundingThis study and medical writing support were funded by QIAGEN Manchester Ltd., Manchester, UK.
Consent statementAll subjects provided informed consent. Study protocol and documentation were approved by an independent Institutional Review Board. The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Author contributionsAll authors made significant contributions to the work reported; took part in drafting, revising and critically reviewing the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
We would like to thank the participants of this study. Medical writing support for the development of this manuscript, under the direction of the author, was provided by Bonnie Nicholson, PhD, of Ashfield MedComms, an Inizio company, and funded by QIAGEN Manchester Ltd, Manchester, UK.






![Comparison of QFN SARS-CoV-2 antigen tube (Nil subtracted) T-cell mediated immune response in subjects pre- and post-booster vaccination (whole blood specimens collected at 0 months [≤7 days], 1 month [18–32 days], and 3 months [81–96 days] following the booster). Wilcoxon matched-pairs signed rank test was used to compare pre-booster and 3-month post-booster responses. Dots represent individual test points and columns represent median responses within the test cohort. Not all subjects had specimens collected at all timepoints. Ag, antigen tube; IU, international units; m: months; ns: non-significant.](https://static.elsevier.es/multimedia/25310437/0000002900000002/v1_202303021319/S2531043722002185/v1_202303021319/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)




