Endobronchial Ultrasound (EBUS) has emerged as a crucial tool for diagnosing intrathoracic disorders, particularly in the staging of lung cancer. However, its diagnostic capabilities in the context of benign and rare diseases remain a subject of debate.
Aimto investigate the diagnostic yield and safety of EBUS-transbronchial mediastinal cryobiopsy (EBUS-TMC) in comparison to EBUS-transbronchial needle aspiration (TBNA) for a broad spectrum of intrathoracic diseases.
Methodsa single-centre retrospective observational study conducted on 48 patients who underwent both EBUS-TBNA and endobronchial ultrasound-transbronchial mediastinal cryobiopsy (EBUS-TMC) in the same procedure between August 2021 and October 2023.
ResultsThe overall diagnostic yield of EBUS-TMC surpassed that of EBUS-TBNA (95.8% vs 54.1 %), notably excelling in the diagnosis of sarcoidosis (92.8% vs 78.5 %), rare mediastinal disorders (100% vs 0 %), hyperplastic lymphadenopathy (100% vs 0 %), and lymphoproliferative disease (100% vs 0 %). No significant differences were observed in the diagnosis of NSCLC and SCLC. Samples obtained through EBUS-TMC facilitated the acquisition of NGS and immunohistochemical analyses more readily.
ConclusionEBUS-TMC may contribute to the precise diagnosis and subtyping of mediastinal diseases, especially lymphomas and rare mediastinal tumors, thereby reducing the number of non-diagnostic procedures.
Disorders arising in the mediastinum or in contact with large conducting airways may have a definite diagnosis through endobronchial ultrasound fine-needle aspiration (EBUS-FNA).1 Due to its excellent sensitivity, specificity, and accuracy, EBUS is considered the procedure of choice for diagnosing lymph node metastasis and for mediastinal staging of non-small cell lung cancer (NSCLC), as recommended by current guidelines.2,3
However, in cases of benign or malignant disorders with a complex histopathologic background, such as lymphoma, Castleman's disease, or rarer entities, the limited amount of material retrieved by needle aspiration may hinder more advanced investigations (such as immunohistochemistry and molecular analyses), which are useful for achieving a confident and definite diagnosis.4–8 Additionally, a significant quantity of tissue retrieval is advisable for epithelial cancers because Next Generation Sequencing (NGS) analysis has become a standard step, especially in adenocarcinoma.9,10
To date, in the pursuit of more comprehensive tissue acquisition, the integration of EBUS with cryobiopsy techniques is emerging as a cutting-edge approach,11 offering a minimally invasive yet effective method to obtain larger and intact tissue specimens from the mediastinum.12–15
The purpose of this study is to report data collected in our cohort of patients who had undergone EBUS-guided cryobiopsy and to analyse which entities could benefit most from this approach.
Materials and methodsThis is a single-centre retrospective observational study conducted on patients who underwent both EBUS-TBNA and endobronchial ultrasound-transbronchial mediastinal cryobiopsy (EBUS-TMC) in the same procedure between August 2021 and October 2023 at the Pulmonology Unit of GB Morgagni - L. Pierantoni Hospital, Forlì. A total of 48 patients were retrospectively evaluated. The following clinical data were extracted from the electronic medical records: age, gender, past medical history, and chest CT scans. All patients were above 18 years of age, and written informed consent was obtained from each patient.
All 48 patients underwent EBUS (BF-UC180F; Olympus Medical Systems, Japan) while being intubated with a rigid tracheoscope (Karl Storz GmbH, Tuttlingen, Germany) and maintained spontaneous breathing. Informed written consent was always obtained from all patients. In the same procedure, EBUS-FNA was performed using a 22G needle (NA-U401SX; Olympus Medical Systems, Japan), and EBUS-TMC was performed using a 1.1-mm flexible cryoprobe (Erbecryo 20402-401, Tübingen, Germany).
Both TBNA and TMC procedures were performed under real-time ultrasound guidance from the EBUS bronchoscope, and the sampling was directed based on the mediastinal lymphadenopathy or masses identified on the chest CT scan. After the initial puncture with a 22G EBUS-TBNA needle, the 1.1-mm cryoprobe was introduced into the working channel of the EBUS bronchoscope. The probe was gently guided to the site within the mediastinum, and it was advanced through the same targeted site created by the 22G EBUS-TBNA needle with real-time ultrasound guidance from the EBUS bronchoscope. The position of the cryoprobe was confirmed within the mass or lymph node station. The cryoprobe was then cooled for 6 s, and the scope unit with the probe inside was withdrawn. The obtained specimens were thawed in saline and fixed in formalin. Pathological investigations were conducted using routine stains (Hematoxylin & Eosin). Immunohistochemical tests and NGS analysis were conducted on the samples that contained a higher amount of valid material, as indicated by the pathologist.
ResultsA total of 48 patients who underwent both EBUS-TBNA and endobronchial ultrasound-transbronchial mediastinal cryobiopsy (EBUS-TMC) procedures in the same session were evaluated. The median age was 60 years (range: 29–80), with 33 (69 %) male patients and 12 (31 %) female patients. Smokers or former smokers were 28 (59 %). Table 1 provides descriptions of the lymph-node stations and sites where masses were detected, along with details of NGS/ICH procedures.
Diagnosis obtained using EBUS-TBNA and EBUS-TMC procedures, and the LFN/mass stations where samples were taken. Lymphnode stations are expressed numerically, as is the amount of NGS/IHC/PD-L1 performed by which procedure.
The median short-axis diameter of the lymph nodes/masses based on chest CT scans was 34 mm (range: 13–74). The diagnoses and diagnostic yields of both approaches are reported in Tables 2 and 3. Because in this study the diagnosis of hyperplastic lymphadenitis was considered valid only if specific morphologic criteria (based on the recognition of lymph node architecture) were identifiable the overall diagnostic yield of EBUS-TBNA was 54.1 %, while it was 95.8 % for EBUS-TBC. Among the patients, 11 were diagnosed with NSCLC through EBUS-TBNA, including 10 cases of adenocarcinoma and 1 of squamous carcinoma. Similarly, EBUS-TBC diagnosed 11 cases of NSCLC, with 10 being adenocarcinoma and 1 being squamous carcinoma. One patient with adenocarcinoma was exclusively diagnosed via EBUS-TBNA, as EBUS-TBC did not reveal neoplastic cells. Conversely, another case was diagnosed solely with EBUS-TBC. Regarding NSCLCs, both EBUS-TBNA and EBUS-TMC exhibited a diagnostic yield of 91.6 %. PD-L1 expression was assessed through immunohistochemistry in 9 cases diagnosed with adenocarcinoma, with 8 cases using cryobiopsy samples and 1 case using EBUS-FNA cell block slides. In the case with a diagnosis of squamous cell carcinoma, PD-L1 assessment was conducted only on cryobiopsy samples. Pathologists selected material for Next-Generation Sequencing (NGS) analysis exclusively from cryobiopsy tissue.
Number of diagnoses obtained from EBUS-TBNA and EBUS-TMC, along with the measurement of the median short-axis diameter based on chest CT scans, stratified by pathology. Diagnoses are expressed numerically, and LFN/mass size in mm.
Overall and disease-stratified EBUS-TBNA and EBUS-TMC diagnostic yield and complications, calculated taking into account that in none of the 48 cases did either EBUS-TMC or EBUS-TBNA prove to be non-diagnostic. The diagnostic yield of the procedures is expressed as a percentage (%).
Both EBUS-TMC and EBUS-TBNA diagnosed 3 cases of neuroendocrine tumors. PD-L1 assessment was exclusively performed using EBUS-TMC. Both procedures equally diagnosed 1 case of mediastinal metastasis originating from kidney cancer.
EBUS-TBNA detected non-necrotizing granulomas in 11 patients, while EBUS-TBC identified them in 13 patients, facilitating a confident diagnosis of sarcoidosis. The diagnostic yield for this benign condition was 78.5 % with EBUS-TBNA and 92.8 % with EBUS-TMC. Concerning mediastinal lymphoproliferative disease, EBUS-TMC successfully diagnosed Hodgkin Lymphoma in 2 cases, whereas TBNA did not. EBUS-TMC enabled the diagnosis of other conditions, including mesothelioma (1 case), pulmonary artery intimal sarcoma (1 case), lung carcinoid (1 case), Castleman disease (2 cases), hamartoma (1 case), silicosis (2 cases). None of these cases received a confident diagnosis through cytology/cell block histology-TBNA.
Furthermore, EBUS-TMC allowed the diagnosis of 8 cases of hyperplastic lymphadenitis; concurrent EBUS-TBNA demonstrated the presence of lymphocytes.
A total of 17(35 %) patients had previously undergone non-diagnostic EBUS-TBNA. The second TBNA was positive in 8 out 17 (3 non-necrotizing granulomas, 2 adenocarcinoma, 2 neuroendocrine tumors, 1 lung carcinoid). EBUS-guided cryobiopsy was diagnostic in 16 out of 17.
EBUS-TBNA failed to provide a diagnosis in 22 procedures (45.8 %), while EBUS-TBC was unsuccessful in 2 procedures (4.1 %).
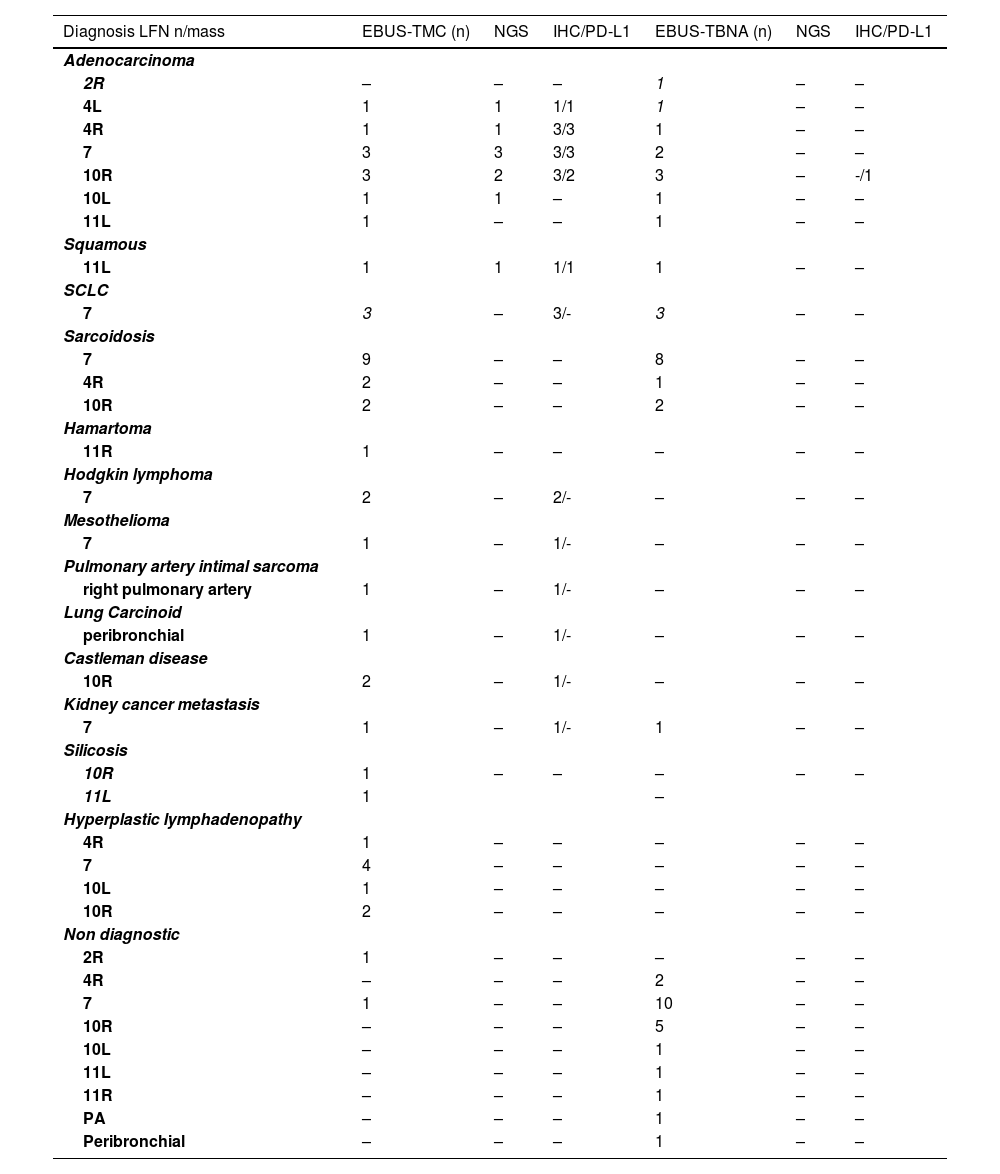
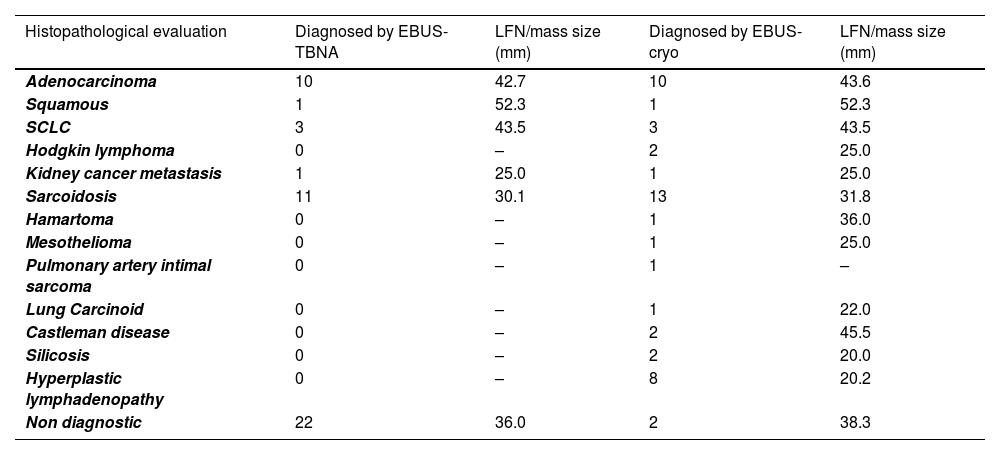
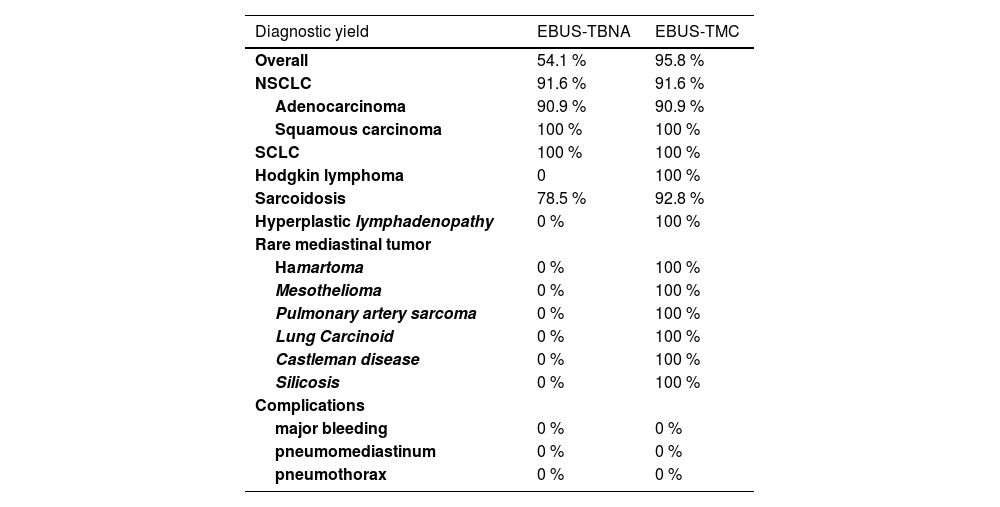
Diagnosis of a classical variant of nodular sclerosis of Hodgkin lymphoma, was feasible with cryobiopsy because the "inflammatory/fibrotic background" in which typical Reed-Sternberg cells depicted by CD15, CD30, PAX5 were detectable, and they were negative for CD20, CD5, CD45, EBV, BOB1, OCT2. None of these details were present in the cell block slides or cytological smears obtained by EBUS-FNA. Intimal pulmonary artery sarcoma was definitively diagnosed using cryobiopsy samples because of the atypical spindle cells embedded in a myxoid stroma showing nuclear expression of MDM2. Morphological and immunohistological features diagnostic of Castleman's disease were present in cryobiopsy samples: lymphoid follicles with regressed germinal centers, prominent vascularization with a few vessels penetrating germinal centers, and a network of cells expressing CD21+, CD23+ markers (qualifying these cells as dendritic cells) (Fig. 1). Hyperplastic lymphadenitis was a diagnosis that could only be made using large specimens obtained by EBUS-TMC, as specific findings such as germinal centers and hyperplasia of parafollicular regions were evident only in the specimens obtained in this manner. Of the 8 patients, 1 received subsequent surgical histological confirmation after a thoracotomy procedure, 6 showed the disappearance of lymphadenopathies during radiological follow-up, and 1 died due to a rapid progression of a pre-existing hematological disorder.
Lymph node tissue in the cryosample (H&E,low power) (a). A “burn-out” or regressed germinal center with a penetrating vessel with thicked wall and high endothelial cells (“lollipop” appearance) (H&E, high power) (b). Numerous germinal centers are highlighted by anti-CD20 monoclonal antibodies (CD20, low power) (c). Large dysplastic cells inside the germinal center marked by CD23 antibody (dendritic intrafollicular cells) (CD23, medium power) (d).
In none of the 48 cases did either EBUS-TMC or EBUS-TBNA prove to be non-diagnostic, enabling the calculation of the diagnostic yield in this patient cohort.
There were no adverse events such as major bleeding, pneumomediastinum, or pneumothorax.
DiscussionOur study demonstrates that the diagnostic yield of mediastinal cryobiopsy in mediastinal lesions or disorders adjacent to large airways is higher compared to that observed with EBUS-TBNA. This increased diagnostic yield is primarily attributed to the diagnosis of lymphoproliferative disorders, rare tumors, or benign entities. Immunohistochemistry and NGS were successfully performed on material obtained by the cryoprobe. Furthermore, the safety profile of mediastinal cryobiopsy is comparable to what has been observed with EBUS-FNA.
Mediastinal masses and lymphadenopathies can present a diagnostic challenge in clinical practice. While EBUS-TBNA offers exceptional diagnostic accuracy for malignancies,16 the limited amount of tissue it retrieves may be inadequate for diagnosing lymphoproliferative diseases and rare tumors.17 These conditions often require histopathological samples rather than cytological ones to assess the overall architectural background.
The presence of small lymphocytes in FNA preparations may suggest a diagnosis of hyperplastic lymphadenatis. However, the final diagnosis requires lymph node tissue with architectural preservation. In our study, this preservation was achieved using cryobiopsy samples.
Additionally, rare mediastinal tumors, which are frequently misdiagnosed, can result in delayed diagnoses and inappropriate management. For patients diagnosed with NSCLC, both techniques have shown equal effectiveness in obtaining tissue for diagnosis. However, NGS analysis was exclusively performed on samples obtained through TMC, primarily due to the larger specimens acquired compared to cytology-driven TBNA samples. Nevertheless, there was one case of NSCLC that was exclusively diagnosed through TBNA.
Among these NSCLC patients, four had previously undergone standalone EBUS-TBNA procedures. In two of these cases, TBNA sampling was non-diagnostic, and in all four cases, the cytological material collected was insufficient for conducting NGS analyses.
In line with Zhang and colleagues' findings,12 the diagnostic yield of EBUS-TMC in NSCLC and mediastinal metastasis from other tumors is comparable to the already high diagnostic yield of EBUS-TBNA. However, cryobiopsy reduces the number of non-diagnostic procedures and, most importantly, enables the acquisition of sufficient samples for conducting NGS analyses.18
Unlike lung cancer staging, the diagnostic accuracy of EBUS-TBNA for primary lymphoproliferative diseases is still debated. Plones and colleagues demonstrated that lymphomas require histological specimens rather than TBNA-acquired cytology for diagnosis and confirmation of the lymphoma subtype.19 This is due to the limited sampling of core tissue with TBNA, as lymphoma diagnosis necessitates multidirectional analysis involving cytology, immunophenotyping, and histology.20
In our cohort, only EBUS-TMC allowed us to perform immunohistochemistry and diagnose two cases of mediastinal Hodgkin Lymphoma. Once again, these two patients had previously undergone non-diagnostic EBUS-TBNA procedures.
The clear superiority in terms of diagnostic yield of EBUS-TMC is evident in the context of rare mediastinal disorders, such as intimal pulmonary sarcoma, mesothelioma, lung carcinoid, Castleman disease, and hyperplastic lymphadenathis (Table 3), confirming the data published in Zhang J's randomized trial.12 For these uncommon conditions, the samples obtained through cryobiopsy enable our pathologists to conduct immunohistochemistry analyses, facilitating accurate disease classification and subtype identification. Within the context of these uncommon conditions, EBUS-TBNA remains insufficient for obtaining a diagnosis.
The application of cryobiopsy in pulmonary diagnostics has witnessed notable expansion in recent years. Notably, it is no longer exclusively confined to the diagnosis of diffuse parenchymal lung diseases. Owing to its heightened safety profile,21 its role has extended to encompass the diagnosis of peripheral pulmonary lesions through radial endobronchial ultrasound (R-EBUS) guided transbronchial cryobiopsy. In the systematic review and meta-analysis conducted by Srymaa PB et al., this technique has demonstrated both safety and efficacy, exhibiting a diagnostic yield superior to conventional forceps biopsy. Furthermore, it facilitates immunohistochemistry or molecular analysis, because of the larger size of cryo-samples.22
This superiority of cryobiopsy is also due in obtaining higher-quality specimens, namely those with more preserved cellular architecture and lack of crush artifacts compared to conventional transbronchial forceps biopsy, which helps the pathologist to more easily reach a diagnosis.23
Many rare mediastinal diseases lack established diagnostic criteria or standardized management protocols. EBUS-TMC provides an opportunity to explore the intricate cellular and structural details of rare mediastinal diseases, shedding light on their underlying mechanisms. This knowledge can pave the way for further research, contributing to a deeper understanding of disease progression and potential therapeutic targets.
ConclusionIn the era of precision medicine and individualized treatments, the use of EBUS-TMC may prove to be a game-changing method that contributes to the accurate diagnosis and subtyping of mediastinal diseases, particularly lymphomas and rare mediastinal tumors. This can result in a reduction in the number of non-diagnostic procedures. EBUS-TMC, as a minimally invasive procedure, offers interventional pulmonologists a means to obtain larger and more representative tissue samples from mediastinal structures, assisting in the precise diagnosis of various benign and malignant lung and mediastinal diseases. The capability of EBUS-TMC to provide histological specimens with preserved architecture, combined with real-time imaging guidance, allows for the execution of immunohistochemistry and molecular analyses, thereby enhancing diagnostic confidence and reducing the necessity for more invasive surgical procedures.
When there is a high clinical suspicion of a rare mediastinal tumor or a primary lymphoproliferative disorder, we believe that EBUS-TMC, in combination with EBUS-TBNA, should be considered as the primary diagnostic approach to reduce unnecessary procedures.
The small sample size of our cohort and the retrospective design of this series pose certain limitations; findings from larger samples might offer greater generalizability, and prospective studies could potentially unveil additional characteristics of EBUS-TMC's role as the primary choice in lymphoproliferative and rare mediastinal disorders.
None.
None.