Evaluation of unexplained exercise intolerance is best resolved by cardiopulmonary exercise testing (CPET) which enables the determination of the exercise limiting system in most cases. Traditionally, pulmonary function tests (PFTs) at rest are not used for the prediction of a respiratory limitation on CPET.
ObjectiveWe sought cut-off values on PFTs that might, a priori, rule-in or rule-out a respiratory limitation in CPET.
MethodsPatients who underwent CPET in our institute were divided into two groups according to spirometry: obstructive and non-obstructive. Each group was randomly divided 2:1 into derivation and validation cohorts respectively. We analyzed selected PFTs parameters in the derivation groups in order to establish maximal and minimal cut-off values for which a respiratory limitation could be ruled-in or ruled-out. We then validated these values in the validation cohorts.
ResultsOf 593 patients who underwent a CPET, 126 were in the obstructive and 467 in the non-obstructive group. In patients with obstructive lung disease, forced expiratory volume in 1 second (FEV1) ≥ 61% predicted could rule out a respiratory limitation, while FEV1 ≤ 33% predicted was always associated with a respiratory limitation. For patients with non-obstructive spirometry, FEV1 of ≥ 73% predicted could rule-out a respiratory limitation. Application of this algorithm might have saved up to 47% and 71% of CPETs in our obstructive and non-obstructive groups, respectively.
ConclusionPresence or absence of a respiratory limitation on CPET can be predicted in some cases based on a PFTs performed at rest.
AbbreviationsAUC area under curve body mass index breathing reserve cardiopulmonary exercise test diffusing capacity of the lung for carbon monoxide forced expiratory volume in 1 second functional residual capacity forced vital capacity global lung function initiative heart rate inspiratory capacity milliliter maximal voluntary ventilation negative predictive value pulmonary function tests positive predictive value respiratory exchange ratio receiver operating characteristic residual volume standard deviation total lung capacity alveolar volume dead space to tidal volume ratio minute ventilation max, maximal oxygen consumption peak, peak oxygen consumption
Exertional dyspnea or exercise intolerance is a patient's complaint of an inability to complete a physical task that a normal subject would find tolerable.1 Pathophysiological explanations include inefficient gas exchange due to ventilation-perfusion mismatching (high physiological dead space), low work rate lactic acidosis (e.g., low cardiac output response to exercise), exercise-induced hypoxemia, and disorders associated with impaired ventilatory mechanics, which may occur in combination.2 The most common etiologies for exercise intolerance are cardiac and pulmonary disorders, however pulmonary and cardiac function tests performed at rest cannot reliably determine exercise.3 In case of discrepancy between the degree of the exercise intolerance and the clinical findings in these tests, or when multiple disorders co-exist, a cardiopulmonary exercise test (CPET) may enable the determination of the exercise limiting system.
Unfortunately, CPET is considered to be under-utilized.4 It must be performed in a specialized exercise laboratory which is costly and time-consuming and requires a skilled team in order to choose the best protocol for the test and interpret the results.5–9 Moreover, during a long pandemic, in which major scientific societies recommend selective referral for CPET studies, it is essential to better select patients who can benefit from the test.
It might be expected that physiologic parameters measured at rest can predict the exercise limit, especially in those with a specific pulmonary or cardiovascular disease. Maximal oxygen consumption (V̇o2 max), the value achieved when V̇o2 remains stable despite a progressive increase in the intensity of exercise, is considered the gold-standard measure of aerobic fitness.1 Empirically, resting pulmonary and cardiac tests do not correlate well with V̇o2 max.2,5,8,10–14 Notably, among the routine pulmonary function tests measured at rest, including spirometry, body plethysmography and diffusion capacity for carbon monoxide (DLCO), DLCO seems to correlate best with V̇o2 max and dyspnea.15,16
Although V̇o2 max cannot be reliably predicted by pulmonary function tests (PFTs) our experience suggests that PFTs may predict whether exercise ventilatory limitation is likely to be present, that is – whether or not the breathing reserve (BR) at peak exercise is reduced. In general, individuals with severely reduced lung function are respiratory limited (i.e., have a reduced BR(2)), while those who have normal lung function can be assumed to have no respiratory limitation on exercise capacity (normal BR).
We hypothesized that subjects with only mildly abnormal resting PFTs are unlikely to have respiratory limitation during exercise. On the other hand, patients with severely reduced PFTs are highly likely to have respiratory limitation (low BR) using traditional CPET. In this study, we set out to determine the existence of a priori cut-off values for different PFTs parameters, above and below which ventilatory limitation can be practically ruled-out or ruled-in.
MethodsWe performed a retrospective observational cohort study. Our study population included adult patients who underwent routine clinical CPET at the Pulmonary Institute, Chaim Sheba Medical Center, Tel-HaShomer, Israel, between July 2008 and August 2015. CPET testing in our institute is performed and interpreted according to Wasserman et al.2 CPET was performed with the patients breathing room air and therefore oxygen-dependent patients were not tested, nor were patients judged to be medically unstable (e.g., recent exacerbation of lung disease, syncope or unstable angina). Immediately prior to CPET, all patients completed spirometry, body plethysmography and measurement of DLCO by the single-breath method. Tests were excluded from the study if performed on children under 18 yrs. of age, when data was incomplete or technically flawed, or if judged by an experienced CPET reader to be a submaximal effort. The study was approved by the institutional review board (IRB) of Chaim Sheba Medical Center (approval no. 2526-15-SMC).
The dependent variables studied were selected measurements from PFTs: forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC, inspiratory capacity (IC), total lung capacity (TLC), residual volume (RV), functional residual capacity (FRC), IC/TLC, RV/TLC, and DLCO. These variables represent key aspects of respiratory function: TLC, FVC, FEV1 and IC as measures of ventilatory capacity; FEV1/FVC ratio as an indicator of airflow obstruction; TLC, FRC and FVC as measures of restrictive disorders; RV, IC/TLC and RV/TLC as measures of air trapping; and DLCO as a measure of gas exchange.
PFTs absolute measurements were converted into the predicted values (% predicted) and z-score according to the 2012 Global Lung Function Initiative (GLI)17 for FEV1, FVC and FEV1/FVC; % predicted according to Quanjer et al.18 for RV, TLC and FRC; and % predicted according to Cotes et al.19 for DLCO. RV/TLC and IC/TLC were given as absolute values. Determination of obstruction was made by GLI z-score for FEV1/FVC and defined as z < -1.64.20 We preferred this lower limit of normal (LLN) method of determining obstruction over the fixed ratio method (FEV1/FVC <0.7) as it is more scientifically sound, and correlates better with clinical outcomes.21 We used maximal voluntary ventilation (MVV, liters/minute) and BR (liters/minute) to determine the presence of a respiratory limitation.2 BR was determined by BR=MVV– V̇E peak (V̇E, minute ventilation), where MVV is the maximal voluntary ventilation and V̇E peak is the minute ventilation at peak exercise. MVV was estimated as 40* FEV1.2,5 The definition of ventilatory limitation was BR ≤ 11 L/min2,5 or BR ≤ 15% MVV.5,6
Study outline and data analysisThe entire cohort was divided into two sub-populations, obstructive and non-obstructive. The rationale for this division stems from the different pathological breathing patterns these two sub-populations manifest during exercise. A patient was defined as obstructive according to the above criteria of FEV1/FVC. All other patients were defined as non-obstructive. For each group, CPETs were randomly allocated 2:1 into derivation and validation cohorts, respectively.
Next, we analyzed each dependent variable in the derivation cohorts of each group in order to establish the value above which no respiratory limitation was found (rule-out cutoff), and the value below which all patients tested have a respiratory limitation (rule-in cutoff; for RV and RV/TLC directionality of the abnormalities was reversed).
After establishing the cut-off values for each PFTs parameter, we calculated the proportion of patients who violate those cut-offs in the validation cohorts (i.e., have a respiratory limitation despite PFTs above the rule-out value or do not have a respiratory limitation despite below the rule-in value).
Analyses were performed using SPSS Statistics, version 24 (IBM).
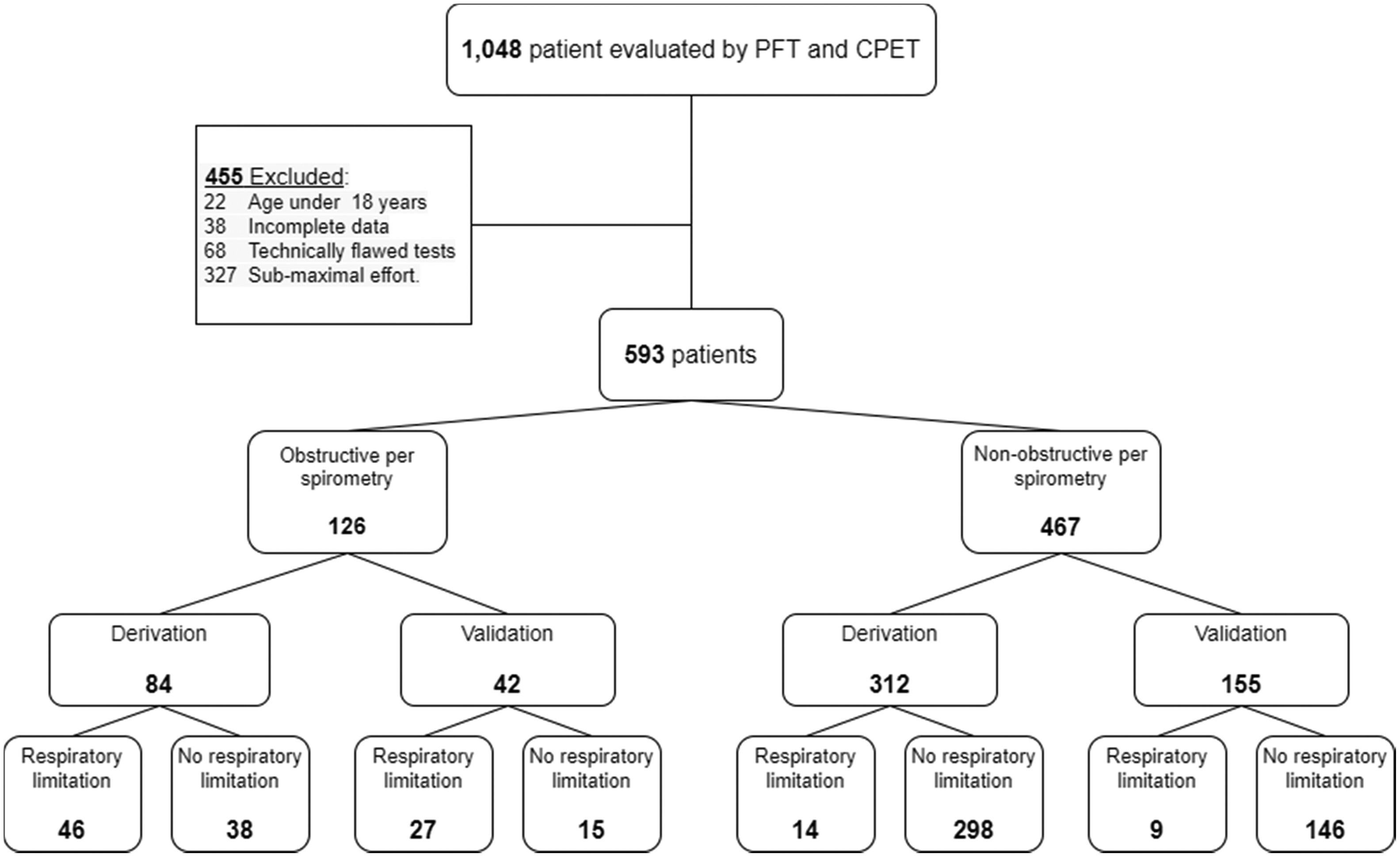
ResultsA total of 1,048 patient underwent CPET at the Pulmonary Institute of Sheba Medical Center. Of these, 455 were excluded (Fig. 1), and the remaining 593 tests were included in this study. Most patients (69%) were referred for CPET to evaluate unexplained exercise intolerance. Other indications included evaluation for heart transplant or other major surgery (18%), evaluation prior to pulmonary rehabilitation (9%), and evaluation of disability (2%). In the remaining 2% of the cases the reason for referral was not stated.
An obstructive spirometry was present in 126 patients, of whom 84 were randomly allocated to the derivation cohort, and 42 to the validation cohort. Non-obstructive spirometry was found in 467 patients, of whom 312 were allocated to the derivation cohort and 155 to the validation cohort.
In the obstructive group, the mean age was 60 ±15 for the derivation cohort and 62 ±12 for the validation cohort (p = 0.874). The mean age of the non-obstructive group was 54 ± 17 for the derivation cohort and 54 ±18 for the validation cohort (p = 0.766).
In the derivation cohort, a respiratory limitation was found in 46 of the 84 (55%) obstructive patients and in 14 of the 312 (5%) patients in the non-obstructive group. In the validation cohort, 27 of the 42 (64%) patients in the obstructive patients (p=0.31 compared to the proportion in the derivation cohort) and 9 of the 155 (6%) patients of the non-obstructive group (p=0.53) had a respiratory limitation.
Obstructive groupAs mentioned above, most patients (55%) in the obstructive group of the derivation cohort had a respiratory limitation (reduced BR; Supplementary material, Table S1). Respiratory limitation was significantly more common among women than among men (71% vs. 45%, respectively, p=0.0225). Height was significantly lower in patients with a respiratory limitation in the derivation cohort (165 ± 9 vs. 171 ± 8 cm, respectively, p<0.001), which could be explained by the higher proportion of women. Body mass index (BMI) was similar. As expected, PFTs showed more severe obstruction and air-trapping in patients with a respiratory limitation. DLCO %predicted was significantly lower in patients with a respiratory limitation in the derivation cohort. Exercise performance as expressed by V̇o2 peak %predicted was similar between patients with and without a respiratory limitation.
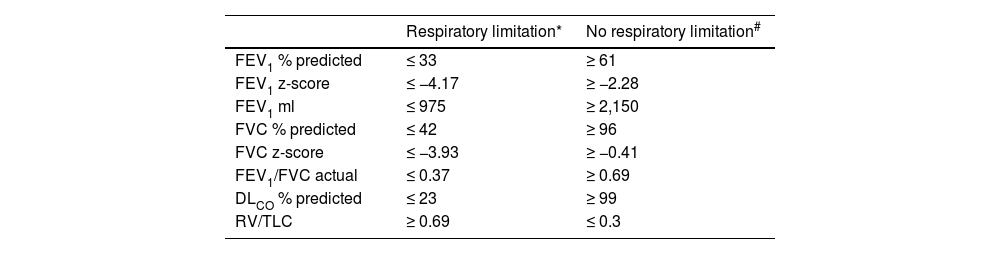
All 19 patients with FEV1 ≤ 33% predicted (z-score ≤ -4.17) had a respiratory limitation in the CPET (Table 1). No patients with FEV1 ≥ 61% predicted (z-score ≤ -2.28) had a respiratory limitation to exercise. The area under curve (AUC) in the receiver operating characteristic (ROC) for FEV1 % predicted was 0.904.
Prediction of a respiratory limitation according to pulmonary lung function test for patients with an obstructive lung disease in the derivation cohort.
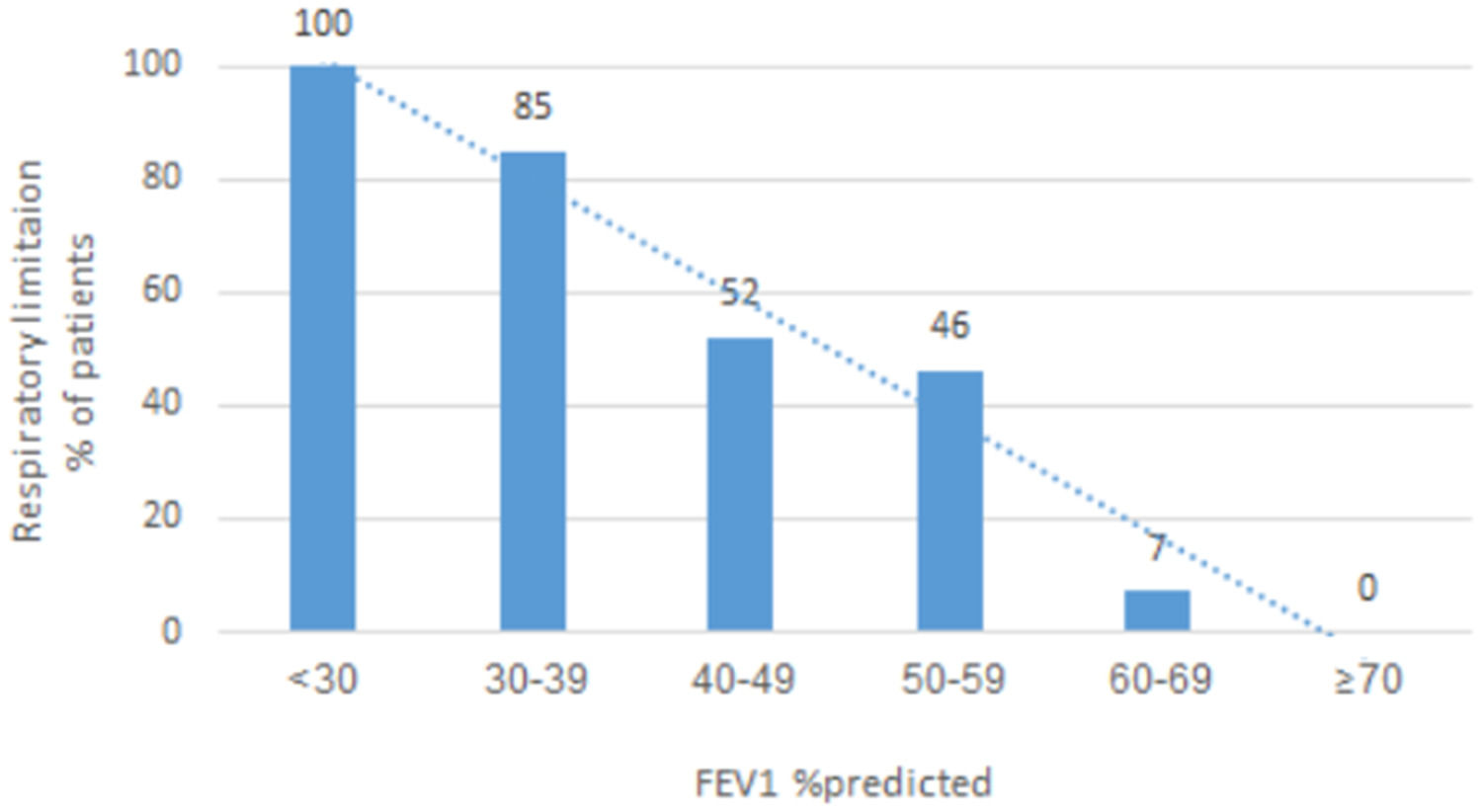
In the validation cohort, all patients with FEV1 ≤ 33% predicted or z-score ≤ -4.17 had a respiratory limitation, while none of the patients with FEV1 ≥ 61% predicted were found to have a respiratory limitation. Fig. 2 shows how the likelihood of having a respiratory limitation on CPET (BR as an indicator for a respiratory limitation) is affected by FEV1 at rest.
Using a cut-off of FEV1 ≤ 33% predicted to rule-in a respiratory limitation would obviate the need for CPET in 32 of the 126 tests (25%) in our obstructive group. Using a cut-off of FEV1 ≥ 61% predicted to rule-out a respiratory limitation would avoid unnecessary CPET in 28 of the 126 tests (22%) in our obstructive group. Hence, 47% of the tests could potentially have been avoided using our upper and lower cut-offs for respiratory limitation based of FEV1 % predicted.
We also analyzed the absolute FEV1 in milliliters (ml) in order to provide the volume range of respiratory limitation and found that all patients with FEV1 ≤ 975 ml had a respiratory limitation while none of the patients with FEV1 ≥ 2,150 ml had respiratory limitation. The AUC for the FEV1 in ml in the ROC was 0.934.
Among the other PFTs parameters that we examined, all patients with either FVC ≤ 42% predicted, FEV1/FVC (actual) ≤ 0.37, DLCO ≤ 23% predicted or RV/TLC ≥ 0.69 had a respiratory limitation on CPET. On the other hand, no patient with FVC ≥ 96% predicted, FEV1/FVC (actual) ≥ 0.69, DLCO ≥ 99% predicted or RV/TLC ≤ 0.3 had a respiratory limitation on CPET (Table 1). In the validation group, all patients with values below the FVC or DLCO and above RV/TLC lower cut-offs had a respiratory limitation, while no patients with values above the upper cutoffs had respiratory limitation.
We did not find clinically relevant cutoff values for discriminating respiratory limitation for RV % predicted, TLC % predicted, FRC % predicted or IC/TLC due to a very wide distribution of the test results.
Non-obstructive groupOverall, ventilatory limitation was very infrequent in the non-obstructive group, present in 23 of 467 (4.9%) of the combined derivation and validation cohorts. Clinical data available was limited, but at least 10 (43%) of these were diagnosed with usual interstitial pneumonia (UIP)-pattern interstitial lung diseases. In PFTs, a restrictive pattern was present in 22 (96%) of these patients (4 very severe, 12 severe, 3 moderately severe, 2 moderate and 1 mild), combined with low DLCO in 20 of them (3 mildly reduced, 11 moderately reduced, 6 severely reduced).
In the derivation cohort of the non-obstructive group, 14 patients (5%) had a respiratory limitation (Supplementary material, Table S2).
The BMI in this sub-group was significantly higher in patients with a respiratory limitation than in patients without a respiratory limitation (30 ± 5 vs. 27 ± 5, respectively, p=0.023). Patients with a respiratory limitation had lower lung volumes and lower DLCO than patients without a respiratory limitation.
No patients with FEV1 ≥ 73% predicted or z-score ≤ -1.7 (226 patients) had a respiratory limitation. In terms of absolute values, no patients with FEV1 ≤ 2,825 ml had a respiratory limitation. However, a lower cutoff, below which all subjects have respiratory limitation, could not be found.
Similar results were found for FVC. No patients with FVC ≥ 71% predicted or z-score ≥ -1.85 (235 patients) had a pulmonary limitation on CPET, but a low cutoff value could not be established. The AUC under the ROCs were 0.965 for FEV1 and FVC, % predicted of the non-obstructive group.
We could not find clinically meaningful cutoff values for respiratory limitation for any other s parameters that were tested in the non-obstructive group.
In the non-obstructive validation cohort only one of the 156 patients (< 1%) a 35-year-old female with primary pulmonary hypertension, had a respiratory limitation with FEV1>73% predicted and FVC > 71% predicted.
Using a cut-off of FEV1 ≥ 73% predicted to rule-out respiratory limitation would eliminate the need for CPET in 330 of 467 patients (71%) in our non-obstructive group (provided this was the reason for CPET referral).
DiscussionIn this study we sought cut-off values on PFTs that might, a priori, rule-in or rule-out a respiratory limitation in CPET. We found that, in patients with an obstructive defect on spirometry, a respiratory limitation of exercise capacity can be ruled-out in patients with whose FEV1 is greater than 61% predicted and can be assumed to be present in patients with FEV1 ≤ 33% predicted. For FEV1 % predicted at the range of 33-61% we provide an estimate of the likelihood of respiratory limitation (Fig. 2).
We also found similar cut-off points for FVC % predicted, DLCO % predicted and for RV/TLC, however these appear to be less clinically useful.
We validated our cut-off values in a separate cohort found them to be fully accurate.
In the non-obstructive group, we found an upper limit capable of ruling out respiratory limitation for FEV1 and FVC (73% and 71% predicted respectively). A lower cutoff for determining that respiratory limitation always be present could not be identified for the non-obstructive group.
Patients with non-obstructive PFTs are very diverse and constitute most of the patients in our CPET database (79%). Only 5% of these had a respiratory limitation on CPET, all but one of whom had a restrictive pattern on pulmonary function tests, usually with concurrent reduced DLCO. Many of the non-obstructive group in our database (146, 31%) had normal PFTs. This might explain both the small proportion of patients with respiratory limitation in our non-obstructive group, and our difficulty in identifying a cutoff for ruling in respiratory limitation a priori. In a study of 15 patients with interstitial lung diseases Agusti et al. found that DLCO/VA (VA - alveolar volume) correlated with ventilation/perfusion (V/Q) mismatch, oxygen diffusion limitation and with the increase in pulmonary vascular resistance elicited by exercise.22 We were unable to identify clinically useful cutoffs of DLCO for predicting the presence or absence of ventilatory limitation in our non-obstructive group. DLCO/VA was not captured in our data base and therefore was not analyzed in our study.
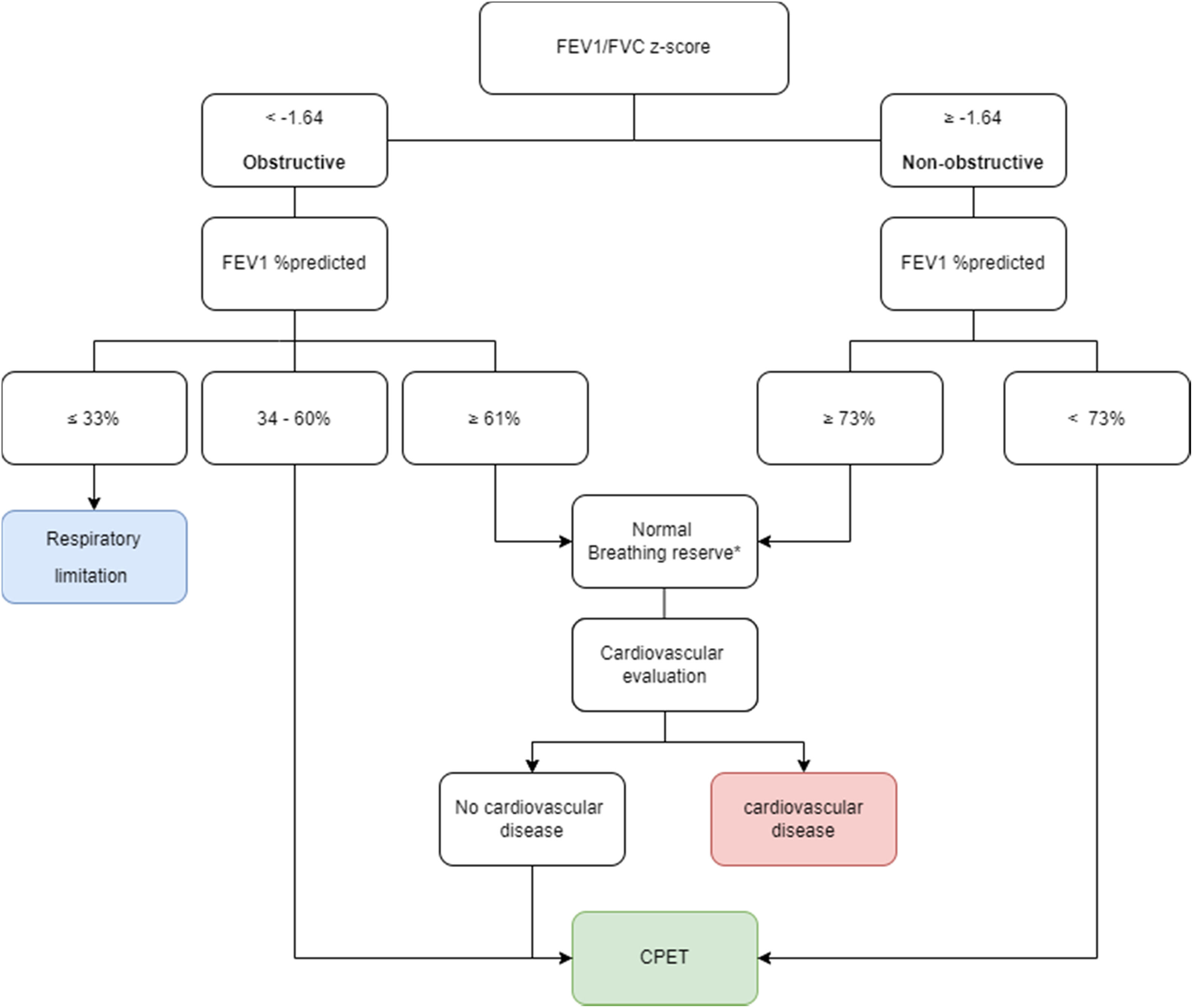
To the best of our knowledge, this is the first study to define cut-off values to a priori rule-in or rule-out a respiratory limitation based on PFTs at rest. According to our findings, we suggest a new algorithm for evaluation of exertional dyspnea (Fig. 3) that might better select patients for CPET. Patients with obstructive lung disease with FEV1 34-60% predicted are reasonable candidates for CPET to evaluate whether the respiratory system is responsible for the exercise intolerance. Application of this algorithm might have saved up to 47% and 71% of CPETs in our obstructive and non-obstructive groups, respectively. Notably, there might be other reasons for a patient with lung disease to be referred for CPET, for example – evaluation of disability, or to guide an exercise prescription for a rehabilitation program. However, in our experience patients with lung disease are commonly referred for CPET in order to determine the exercise-limiting physiological system, and it is these patients, and the physicians who refer them for CPET, who will most benefit from our work.
Our study has several limitations. First, this study was performed retrospectively in a single tertiary center and should be externally validated. Second, the small number of restrictive patients in our database somewhat limits the validity of our findings. Third, CPETs in our database were performed with a cycle ergometer, and results may not be generalizable to other ergometers (e.g., treadmill) or test protocols. Finally, the definition of respiratory limitation is not well established. Different criteria to determine the presence of respiratory limitation might have changed the results of our study. We defined respiratory limitation as the presence of either one of the two most commonly recommended criteria - if BR is 11 L/min or less2,5 or if BR is 15% of MVV or less.5,6 Moreover, in patients with obstructive lung disease measurement of dynamic respiratory mechanics during exercise can identify patients with respiratory limitation despite having normal BR.2,23–25 Chin et al. demonstrated that in patients with mild COPD, significant dynamic mechanical constraints may be present despite a normal BR.26 However, these methods for tracking dynamic hyperinflation are more complex to perform and interpret. Therefore, since all patients with obstructive lung disease with FEV1 ≤ 33% predicted have abnormal breathing reserve, complex CPET protocols to determine respiratory limitation can be avoided.
In conclusion, a respiratory limitation on CPET can be ruled in or ruled out, in some cases, according to the PFTs at rest, with good accuracy. Further studies are needed to confirm our findings.
Funding sourceNone.
Ethics committee approvalThe study was approved by the institutional review board (IRB) of Chaim Sheba Medical Center (2526-15-SMC).
CRediT authorship contribution statementD. Shlomi: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. T. Beck: Formal analysis, Investigation, Methodology, Writing – original draft. R. Reuveny: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – review & editing. M.J. Segel: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.