Obesity hypoventilation syndrome (OHS) is an undesirable consequence of obesity, defined as daytime hypoventilation, sleep disorder breathing and obesity; during the past few years the prevalence of extreme obesity has markedly increased worldwide consequently increasing the prevalence of OHS. Patients with OHS have a lower quality of life and a higher risk of unfavourable cardiometabolic consequences. Early diagnosis and effective treatment can lead to significant improvement in patient outcomes; therefore, such data has noticeably raised interest in the management and treatment of this sleep disorder. This paper will discuss the findings on the main current treatment modalities OHS will be discussed.
Obesity hypoventilation syndrome (OHS) is a disorder characterised by obesity (BMI (body mass index)>30), daytime hypercapnia and sleep-disordered breathing including severe or moderate obstructive sleep apnea (OSA), combined OSA and hypoventilation, or isolated OHS; after excluding other etiologies of hypoventilation.1 See Table 1. Around 90% of patients with OHS have related obstructive sleep apnea (OSA)2 with 73% having severe OSA.3 Only 10% of patients with OHS do not have OSA but rather have non-obstructive sleep hypoventilation.4–6 OHS is prevalent, and if untreated, can lead to significant adverse outcomes, increasing risk of hospitalisation and death,7–12 likely because of respiratory and cardiovascular complications.12–15 The prevalence of OHS is likely to increase globally with the increasing prevalence of severe obesity.16–18
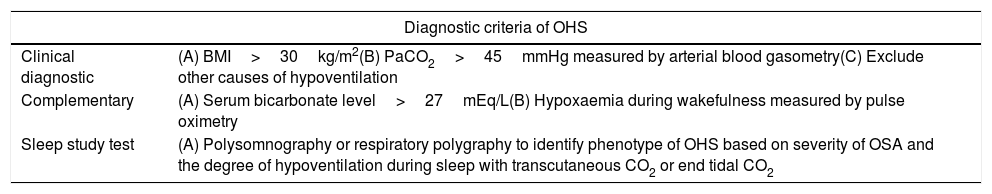
Diagnostic criteria of OHS. The OHS diagnosis is clinical; the complementary findings could be present or not in all OHS patients, so are not necessary to establish the diagnosis of OHS.
| Diagnostic criteria of OHS | |
|---|---|
| Clinical diagnostic | (A) BMI>30kg/m2(B) PaCO2>45mmHg measured by arterial blood gasometry(C) Exclude other causes of hypoventilation |
| Complementary | (A) Serum bicarbonate level>27mEq/L(B) Hypoxaemia during wakefulness measured by pulse oximetry |
| Sleep study test | (A) Polysomnography or respiratory polygraphy to identify phenotype of OHS based on severity of OSA and the degree of hypoventilation during sleep with transcutaneous CO2 or end tidal CO2 |
BMI: body mass index; CO2: carbon dioxide; OSA: obstructive sleep apnea; OHS: obesity hypoventilation syndrome; PaCO2: partial pressure of carbon dioxide. Authors: Victor R. Ramírez Molina; Juan F. Masa Jiménez.
Over the past decade, increasing attention has been paid to the evaluation and management of OHS; rising rates of global obesity along with greater awareness of the significant health and social costs of this disorder have been driving factors fuelling interest in how best to manage those with OHS.19
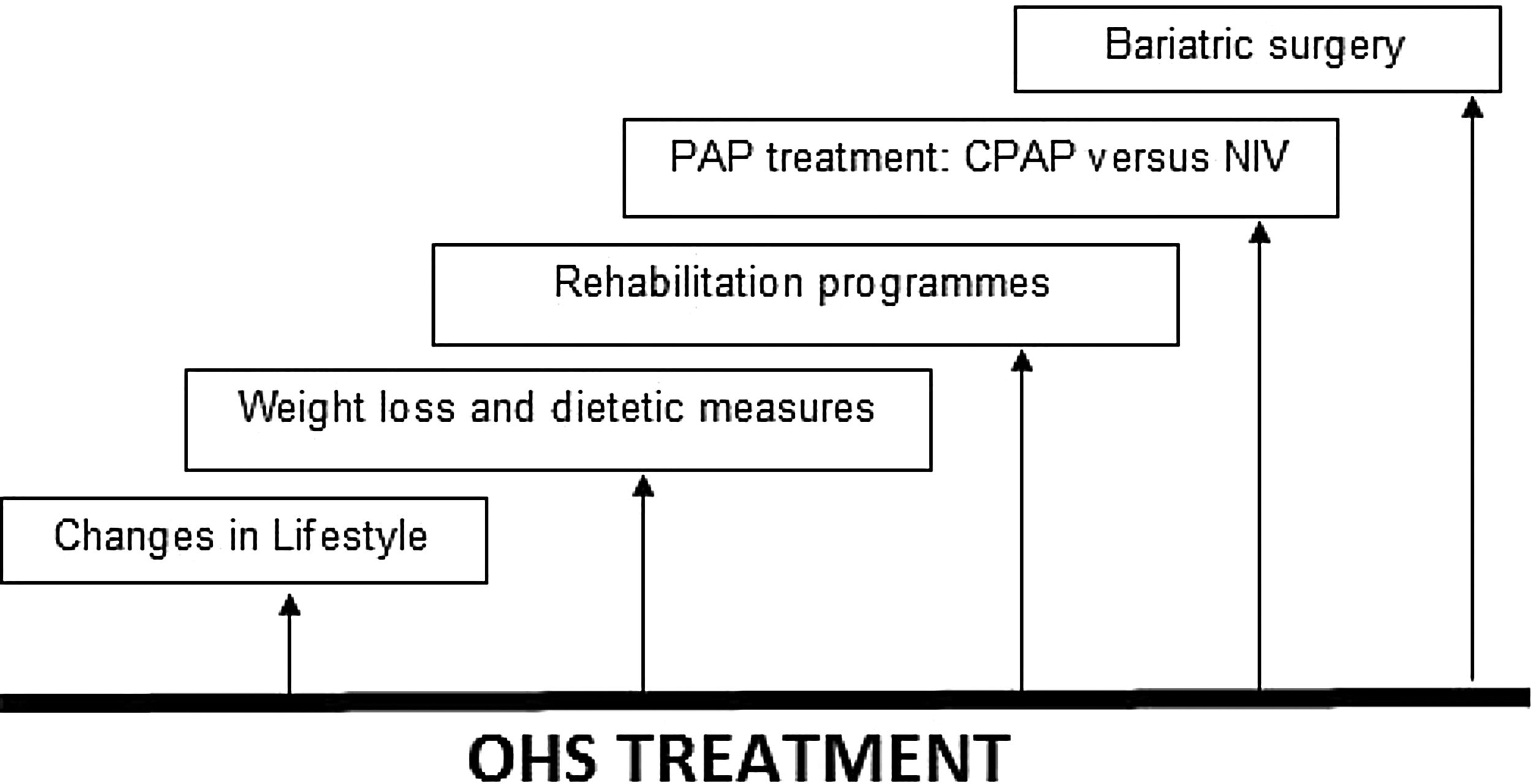
The evaluation, management and treatment of OHS must be multidisciplinary and as such should include expertise from chest physicians, sleep specialists, cardiologists, nutritionists and/or bariatricians; most treatment strategies focus on treating sleep-disordered breathing (SDB) with positive airway pressure (PAP) therapy during sleep, as opposed to aiming to reduce cardiovascular risk profile, also include lifestyle changes, weight loss, bariatric surgery and rehabilitation programmes.20 See Fig. 1.
Treatment in OHS. OHS must be treated multidisciplinary, including lifestyle changes, dietetic measures and weight loss, rehabilitation programmes, PAP treatment (CPAP or NIV) and bariatric surgery (especially for younger patients). Abbreviations: CPAP: continuous positive airway pressure; NIV: noninvasive ventilation; PAP: positive airway pressure; OHS: obesity hypoventilation syndrome. Authors: Victor R. Ramírez Molina; Juan F. Masa Jiménez.
In this review we will explore treatment of OHS in the different modalities, with special attention on PAP treatment.
Weight loss and bariatric surgeryDespite appropriate adherence to PAP therapy, multiple studies have shown that cardio-metabolic risk factors of severe obesity are persistent,3,21,22 with also occurs with cardiovascular morbidity and mortality, remaining high in patients with OHS.23–25
Weight loss continues to be the ideal treatment, as it has been proven to improve diurnal respiratory failure, pulmonary hypertension, sleep-disordered breathing as well as improvements in cardiovascular and metabolic outcomes.4,26 It has been suggested that loss of 25–30% of actual body weight can lead to the resolution of the OHS.20,26 However, it is difficult to achieve and maintain this degree of weight loss without bariatric surgery.26 When compared to non-surgical methods of weight loss, bariatric surgery has proven to be much more effective for sustained weight loss in patients who suffer from severe obesity (BMI>40kg/m2).
Bariatric interventions are effective in achieving significant, sustainable, weight loss that can improve cardiovascular and metabolic outcomes. The safety of bariatric procedures has improved over time; most recent clinical trials have reported improvements in metabolic and cardiovascular morbidities and reductions in all-cause and cardiovascular mortality in patients undergoing laparoscopic sleeve gastrectomy or gastric bypass surgery.26 Laparoscopic sleeve gastrectomy, Roux-en-Y gastric bypass or biliopancreatic diversion with duodenal switch are more likely to lead to the magnitude of weight loss necessary to lead to the resolution of OHS than laparoscopic gastric banding.26
A systematic review and meta-analysis in 201427 examined effectiveness and risks of bariatric surgery; gastric bypass was shown to be the most effective method to date regarding weight loss but has proved to be associated with more complications as well. Data has shown adjustable gastric banding to have a lower mortality and complication rates, yet the reoperation rate presented by the patients was higher and weight loss was less substantial than gastric bypass; sleeve gastrectomy appeared to be more effective in weight loss than adjustable gastric banding and comparable to gastric bypass.
Another systematic review20 examined whether a weight loss intervention should be performed in patients with OHS; the studies found that a comprehensive weight loss programme (including motivational counselling, dieting, and exercise) can reduce weight by 6–7%, but confers no clinically significant effects compared to standard care. Bariatric surgery, on the other hand, is associated with more robust weight loss (15–64.6% depending on the type of intervention), reduction of obstructive sleep apnea severity (18–44% reduction of the AHI (apnea–hypopnea index)), and improvement in gas exchange (17–20% reduction in PaCO2), ultimately leading to the resolution of OHS. Moreover, daytime sleepiness and pulmonary artery pressure also improve with significant weight loss. Bariatric surgery is associated with adverse effects in roughly one-fifth of patients, but serious adverse effects are very rare. The level of certainty in the estimated effects has shown to be very low for most adverse outcomes.
The impact of bariatric surgery on improving OSA using the metric of AHI has presented a high rate in variations. In a meta-analysis of 12 studies, which included a total of 342 patients, Greenburg et al. showed a decrease in the AHI of 55episodes/h to 16episodes/h in patients with OSA and severe obesity; however, many of these patients remained with moderate or worse OSA (AHI>15episodes/h) and therefore continue to require treatment for OSA.28 In patients with OHS, 14% continue to require positive airway pressure treatment after surgical weight loss.29 Despite numerous claims in the lay press that bariatric surgery can cure OSA, several studies have shown that OSA may persist following weight loss. Recurrence or worsening of sleep apnea has been observed following an initial weight reduction even without a concomitant weight increase. The most important predictor of OSA severity following weight loss is the preoperative severity of disease, as measured by the AHI.30,31
There are limited long-term data on the efficacy of bariatric surgery in OHS29 and it is not a safe option for some patients with significantly increased perioperative risk.32 The perioperative adverse events include venous thromboembolism, surgical reintervention, and prolonged hospital stay.33,34
Some degree of weight regain (>15% gain of initial weight loss postbariatric surgery) occurs in 25%–35% of patients 2–5 years after bariatric surgery35–37; the recurrence of OHS with this level of weight gain remains unclear.
The guideline panel of a systematic review in 2020 made a conditional (i.e. weak) recommendation suggesting a weight loss intervention for patients with OHS. This recommendation was based on very low-quality evidence. Although the weight loss target is based upon the observation that greater weight loss is associated with better outcomes, there is a need for better quality studies to ascertain the degree of weight loss necessary to achieve improvement in clinically relevant outcomes in patients with OHS.20
Rehabilitation programmesThe data regarding randomised clinical trials of weight loss targeted rehabilitation programmes for patients with OHS is very limited; pulmonary rehabilitation is an established form of treatment for patients with chronic pulmonary disease, thus similar programmes with particular emphasis on obesity can be expected to benefit patients with OHS. To further reduce the high cardiovascular and metabolic burden in OHS, there is a need for a multimodal a therapeutic approach combining home NIV/CPAP with lifestyle interventions and rehabilitation programmes.38
In a small pilot clinical trial,39 3 months of a multimodal hybrid inpatient-outpatient motivation, exercise and nutrition rehabilitation programme was added to home NIV therapy, compared to just NIV therapy, the addition of this comprehensive rehabilitation programme led to greater weight loss, exercise capacity and improved quality of life at 3 months while the participants were actively enrolled in the 3-month programme; However, these benefits were not sustained at 12 months.
Supplemental oxygen therapyIn approximately 20%–30% of patients with OHS, hypoxaemia during sleep persists despite adequate titration of NIV or CPAP.40 High concentration of supplemental oxygen (i.e. 100% or 50% FiO2) without positive pressure therapy can lead to increased hypoventilation and worsening of hypercapnia in patients with OHS41,42; however, the effect of lower concentrations of supplemental oxygen added to PAP therapy remains unclear. The effect of 2 months of supplemental oxygen therapy added to PAP therapy (CPAP or NIV) was examined in a post hoc analysis of 302 patients.43 In the NIV group, supplemental oxygen was associated with a reduction in the systolic blood pressure. However, the reduction in body weight could have partially confounded this effect. Oxygen added to CPAP was associated with increased frequency of morning confusion. In the lifestyle modification group (i.e. no PAP therapy), supplemental oxygen therapy was associated with compensatory metabolic alkalosis and a decrease in the AHI. In aggregate, 2 months of oxygen therapy was associated with marginal changes that were insufficient to consider it either beneficial or harmful.43 Long-term studies examining the role of oxygen therapy alone or added to PAP therapy are necessary.
In a double-blind, randomised, controlled, crossover trial, 24 outpatients newly diagnosed with OHS inhaled 100% oxygen or room air for 20min on 2 separate days; the study showed that breathing 100% oxygen causes worsening hypercapnia in stable patients with OHS.41 Later, in a double-blind randomised crossover study, OHS patients breathed oxygen concentrations (FiO2 0.28 and 0.50), each for 20min, separated by a 45min washout period. The study investigated the effects of moderate concentrations of supplemental oxygen on PCO2, pH, minute ventilation among people with stable untreated OHS, with comparison to healthy controls. The findings concluded that among people with mild, stable untreated OHS, breathing moderate concentrations of supplemental oxygen increased PaCO2, sufficient to induce acidaemia during FiO2 0.50.42
Positive airway pressure therapyOHS is treated with positive airway pressure (PAP) therapy during sleep. The two most commonly used PAP modalities are continuous positive airway pressure (CPAP) and noninvasive ventilation (NIV). Although CPAP can splint the upper airway open and effectively treat OSA, it does not increase ventilation as effectively as NIV does.44
The short-term benefits of CPAP include improvement in gas exchange and sleep-disordered breathing45 with an observed response between 50% and 80% of cases although it may vary in the sleep apnea severity and the time of follow-up. This improvement is directly proportional to the hours of CPAP use, as each hour of use of PAP therapy decreased the PaCO2 by 1.8mmHg and the PaO2 increased by 3mmHg.40
PAP therapy improves gas exchange, respiratory sleep disorders and probably lung function and central respiratory impulse to carbon dioxide (CO2). Night-time hypoventilation can be effectively improved, but not in all cases,46–49 and daytime PaCO2 reduced or restored to normal values.48 The effectiveness of NIV has been assessed in several long-term, observational studies1,7,23,44,50–55 and medium-term randomised trials.3,21,56
In patients with OHS and severe OSA, medium-term randomised controlled trials7,38,50,57–59 and a long-term clinical trial24,26,60 have shown CPAP and NIV to be equally effective in improving symptoms, quality of life and sleep, gas exchange during waking and sleep, as well as spirometric and polysomnographic parameters.
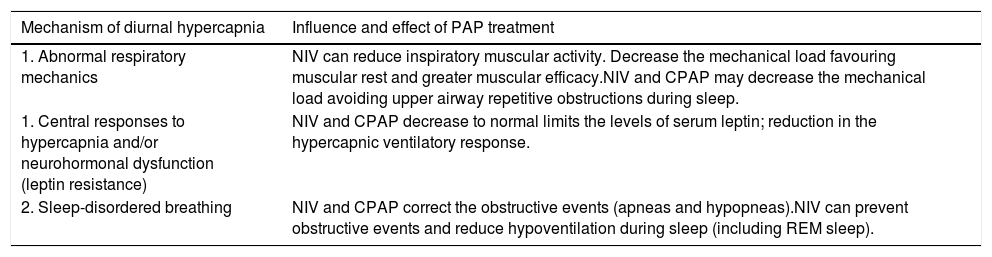
Mechanisms of improvement in hypercapnia with PAP useThe mechanisms by which diurnal hypercapnia improves with PAP are complex and not fully understood. PAP therapy can influence the following mechanisms: abnormal respiratory mechanics, central responses to hypercapnia and/or neurohormonal dysfunction (leptin resistance) and sleep-disordered breathing.61 See Table 2.
Potential mechanism of improvement with PAP therapy in OHS. PAP therapy can influence in the following mechanisms to improve daytime hypercapnia: mechanical load, leptine resistance and breathing sleep disorder; including reduce inspiratory muscular activity favouring muscular rest, decrease in central resistance leptine and decrease in nocturnal obstructive events and sleep hypercapnia.
| Mechanism of diurnal hypercapnia | Influence and effect of PAP treatment |
|---|---|
| 1. Abnormal respiratory mechanics | NIV can reduce inspiratory muscular activity. Decrease the mechanical load favouring muscular rest and greater muscular efficacy.NIV and CPAP may decrease the mechanical load avoiding upper airway repetitive obstructions during sleep. |
| 1. Central responses to hypercapnia and/or neurohormonal dysfunction (leptin resistance) | NIV and CPAP decrease to normal limits the levels of serum leptin; reduction in the hypercapnic ventilatory response. |
| 2. Sleep-disordered breathing | NIV and CPAP correct the obstructive events (apneas and hypopneas).NIV can prevent obstructive events and reduce hypoventilation during sleep (including REM sleep). |
CPAP: continuous positive airway pressure; NIV: noninvasive ventilation; OHS: obesity hypoventilation syndrome; PAP: positive airway pressure; REM: rapid eye movement. Authors: Victor R. Ramírez Molina; Juan F. Masa Jiménez.
NIV can reduce inspiratory muscular activity,62 so that it can efficiently decrease the mechanical load favouring muscular rest and greater muscular efficacy during the day after nocturnal NIV treatment. Continuous positive airway pressure and NIV may decrease the mechanical load avoiding upper airway repetitive obstructions during sleep.
As for leptin resistance, the levels of serum leptin decrease to normal limits in patients with OSA treated with CPAP,63,64 but it is assumed that apneas and hypopneas are the cause of the elevated leptin levels rather than being the result of them.65,66 Leptinaemia also decreases with NIV treatment67,68 as does daytime hypercapnia, and some studies have shown a correlation between leptinaemia and a reduction in the hypercapnic ventilatory response,69 while another study68 reported contradictory results, i.e., an increase of leptin with NIV. Therefore, the role of leptin in how NIV treatment achieves improvement, is still unclear.
Finally, in regards to sleep-disordered breathing, repetitive obstructive events produce increasing hypercapnia, not resolved with the hyperventilation that occurs at the end of obstructive events. Despite correction of these nocturnal obstructive events with CPAP, daytime PaCO2 does not return to normal in all cases. Several studies have highlighted that the CPAP response may vary depending on the predominance of nocturnal obstructive events10,44 and the time of follow-up because CPAP may have a delay in its efficacy related to NIV.22 Non-invasive ventilation has shown to prevent obstructive events and reduce hypoventilation during sleep (including rapid eye movement [REM] sleep). Both NIV and CPAP should decrease nocturnal hypercapnia, leading to lower daytime serum bicarbonate and consequently less blunting of the central carbon dioxide response.70
Scientific research baseThere are several randomised controlled studies that compare different treatments in OHS.3,22,24,56,71 One of these studies compared the short-term efficacy of NIV and CPAP treatments in 36 patients with OHS selected in considering their favourable response to a night of CPAP treatment.71 After 3 months, the improvements in daytime sleepiness and in clinical and gas exchange parameters were similar between CPAP and NIV groups.
In another trial including 38 patients with mild hypercapnia with NIV compared to a control group treated with conservative measures, the NIV group had a significant reduction in daytime PaCO2, bicarbonate and an increase in pH. Therapy with NIV, as expected, was associated with a great improvement in all sleep variables analysed, sleep architecture, average oxygen saturation, oxygen saturation time less than 90%, apnea and hypopnea index, with a positive and significant correlation between average oxygen saturation during sleep and diurnal arterial blood gases. In contrast, no change was observed in any of the metabolic and inflammatory parameters studied, but the follow-up was only one month, so no other conclusions could be drawn.21 In this study, the patients had a lower BMI and were less hypercapnic than the subjects included in other trials.3,71
The Pickwick project is the largest multicenter randomised controlled trial designed to assess medium-term (two months) effectiveness of NIV, CPAP, and lifestyle modification (control group) and long-term (3 years) effectiveness between NIV and CPAP in OHS65,72; there are two clinical trials in parallel depending on the existence or absence of severe OSA (AHI≥30). The trial which includes severe OSA has three arms: NIV, CPAP and change in lifestyle for two months (first phase). After this period of time, the lifestyle change group was re-randomised to NIV or CPAP to continue at least 36 months (second phase). Patients with an AHI<30 were randomised to NIV or change in lifestyle for at least 36 months (second phase), although an evaluation of results was performed at two months (first phase). The results of the first phase were entirely published.3 The first publication included 221 patients with severe OSA randomised to NIV, CPAP and change in lifestyle; PaCO2 who improved with each of the three treatments, but the improvement was greater with the use of NIV, with a significant difference in relation to the group of conservative measures. In the CPAP group, the reduction of PaCO2 depended on compliance with the treatment. Thus, NIV and CPAP decreased blood bicarbonate levels but after adjusting baseline data only NIV achieved statistical significance compared to the control group. Sleep variables improved notably with the use of NIV and CPAP, both proving to be equally efficient and with little or no difference between the two; only the NIV group presented an increase in the FVC, FEV1 values and the 6-minute walk test. In another publication of this Pickwick study, 86 patients were randomised and treated for two months with NIV or lifestyle modifications.56 The NIV group significantly improved PaCO2 and serum bicarbonate levels compared to the control group.
In another randomised controlled trial, NIV or CPAP was used for 3 months with 60 participants. The primary objective was to identify the frequency of treatment failure defined as, hospital admission, persistent ventilatory insufficiency or lack of adherence, while secondary objectives included life quality related to health and drowsiness. A total of 57 patients completed the follow-up without differences in treatment failure between the groups (NIV 14.8% versus CPAP 13.3%; p=0.87). It is worth noting that adherence to treatment and PaCO2 in wakefulness at three months were similar (NIV 5.3h/night; CPAP 5.0h/night; p=0.62, and PaCO2 in wakefulness at three months of 44, 2 and 45.9mmHg, respectively; p=0.60). The differences between the groups in the improvement of sleepiness and the life quality were not significant. Baseline severity of ventilatory failure (based on PaCO2 levels) was the only significant predictor for such insufficiency after a three months periods of the study (OR, 2.3; p=0.03).22
The multicentre, open-label, randomised controlled Pickwick trial published its long-term results on 97 OHS patients with severe OSA treated with NIV and 107 treated with CPAP.24 The median follow-up was 5.44 years (interquartile range [IQR] 4.45–6.37) for all patients, 5.37 years (4.36–6.32) in the CPAP group, and 5.55 years (4.53–6.50) in the NIV group. The hospitalisation days per patient-year were 1.63 (standard deviation [SD] 3.74) in the CPAP group and 1.44 (3.07) in the NIV group (adjusted rate ratio 0.78, 95% CI 0.34–1.77; p=0.561). Changes in other hospital resource utilisation, blood pressure, arterial blood gases, spirometry, quality of life, clinical symptoms and supplemental oxygen therapy remained similar between PAP modalities. Both NIV and CPAP also similarly improved the pulmonary artery pressure and diastolic left ventricular dysfunction.72 Given that CPAP has lower complexity and cost, CPAP might be the preferred first-line PAP treatment modality.24
Cost-effectiveness of PAP therapy modalitiesCPAP is simpler to implement and is less costly than NIV.24 To investigate which of the two treatments is more cost-effective, the only study reported is from Masa and cols, whom carried out a post hoc, within-trial, cost-effectiveness analysis using the large multicentre, open-labelled, randomised controlled study (Pickwick study)3,24,43,56,65,73,74; the aim was to determine the comparative cost-effectiveness relationship between NIV and CPAP based on 3 years of follow-up, using hospitalisation days as the primary outcome measure in a cost-effectiveness analysis, or considering the hospitalisation days in monetary terms where the value of a hospitalisation day is approximately its cost, in a cost–benefit analysis.
In total, 363 patients were selected, 215 were randomised and 202 were available for the analysis. The median (IQR) follow-up was 3.01 (2.91–3.14) years for NIV group and 3.00 (2.92–3.17) years for CPAP. The mean (SD) Bayesian estimated hospital days was 2.13 (0.73) for CPAP and 1.89 (0.78) for NIV. The mean (SD) Bayesian estimated cost per patient/year in the NIV arm, excluding hospitalisation costs, was €2075.98 (91.6), which was higher than the cost in the CPAP arm of €1219.06 (52.3); mean difference €857.6 (105.5). CPAP was more cost-effective than NIV (99.5% probability) because longer hospital stay in the CPAP arm was compensated for by its lower costs. Similar findings were observed in the high and low adherence subgroups; Thus, the conclusion of this study is that CPAP is more cost-effective than NIV; therefore, CPAP should be the preferred treatment for patients with OHS with severe OSA.75
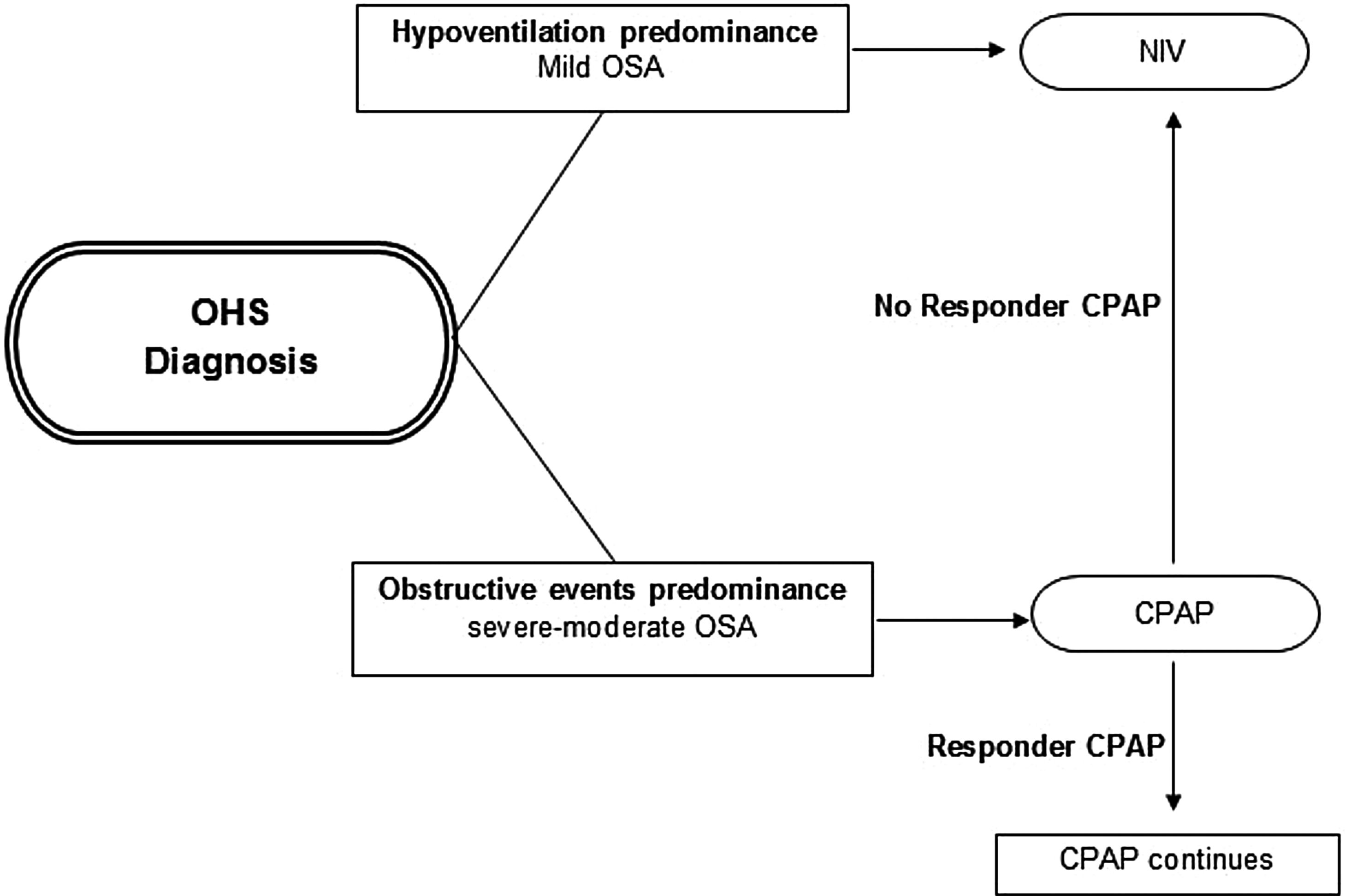
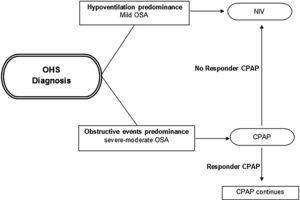
Initial PAP treatment suggestionCPAP should be the initial treatment modality in patients with OHS if severe OSA is present, due to its relative simplicity, low cost and efficacy.4,46,75 In patients with OHS without severe OSA, NIV is the preferred PAP modality, because in these patients without a significant number of obstructive apneas and hypopneas, their nocturnal hypoventilation may depend on other mechanisms (e.g. obesity). See Fig. 2. However, a case by case evaluation is necessary to determine the initial treatment.26
Initial PAP treatment election in OHS. CPAP should be the initial modality treatment if the main cause of diurnal hypoventilation is the predominance of obstructive events (severe–moderate OSA) and if in subsequent evaluations at 1–3 months the patient responds with adequate oxygenation (PaO2) and ventilation (PaCO2) the CPAP should be continued; if the patient does not responds or if the OHS patient has hypoventilation predominance (mild OSA), NIV should be the initial modality treatment preferred. Abbreviations: CPAP: continuous positive airway pressure; NIV: noninvasive ventilation; OHS: obesity hypoventilation syndrome; OSA: obstructive sleep apnea; PaO2: partial pressure of oxygen; PaCO2: partial pressure of carbon dioxide; PAP: positive airway pressure. Authors: Victor R. Ramírez Molina; Juan F. Masa Jiménez.
In acute hypercapnic respiratory failure or hospitalised patients, NIV should be the first option, due to its potentially greater efficacy against hypoventilation and the underlying severity of respiratory failure.61
ConclusionsObesity is a worldwide, increasingly ubiquitous health issue that has triggered the wide spread of several related medical conditions; OHS is one of the respiratory sleep disorders that has the greatest impact on increasing cardiovascular risk. Therefore, OHS must be recognised promptly, and optimally treated; a multidisciplinary approach is likely to be more effective for improving long-term outcomes, including loss weight, rehabilitation programmes and PAP therapy; CPAP should be the initial treatment when indicated.
Authors’ contributionsAll authors equally contributed to this paper with conception and design of the study, literature review, critical revision, editing and final approval of the final version.
Conflicts of interestThe authors declare that they have no conflicts of interest.
None declared.