Humidification and non-invasive ventilation are frequently used together, despite the lack of precise recommendations regarding this practice. We aimed to analyse the impact of active external and built-in humidifiers on the performance of home ventilators, focusing on their pressurization efficacy and their behaviour under different inspiratory efforts.
MethodsWe designed a bench study of a lung simulator programmed to emulate mechanical conditions similar to those experienced by real respiratory patients and to simulate three different levels of inspiratory effort: five different commonly used home NIV devices and active humidifiers attached to the latter (internal or “built-in”) or to the circuit (external). To test ventilator pressurization under different humidification and effort settings, pressure-time products in the first 300ms and 500ms of the respiratory cycle were calculated in the 45 situations simulated. Inferential statistical analysis was performed.
ResultsA significant reduction of PTP 300 and PTP 500 was observed with the external humidifier in three of the devices. The same pattern was noted for another device with an internal humidifier, and only one device showed no significant changes. This impact on pressurization was commonly higher under high inspiratory effort.
ConclusionsThese results indicate the need to monitor pressure changes in the use of external humidification devices in some home NIV ventilators.
Data on the efficacy of non-invasive mechanical ventilation (NIV) in improving breathlessness, arterial blood gases and respiratory drive and its usefulness in preventing the need for intubation in many cases is currently undeniable.1 A large number of patients benefit from this therapy during our daily clinical practice. Despite the absence of high-quality evidence on its use and efficacy, humidification during therapy with NIV is widespread, both in the acute care setting and in home care.
The need for air humidification is widely accepted in invasive mechanical ventilation to prevent harmful effects on respiratory airways and to preserve normal respiratory function.2 However, the importance of this measure has been traditionally overlooked in NIV, which has been considered to respect the physiological humidification of air in the upper airways. However, physiological conditioning of air could become insufficient with NIV, as the air delivered by the ventilator is colder and dryer than ambient air, as a result of the common presence of leaks in the NIV circuit and the high unidirectional flow by which this air is delivered.3 Although upper airways are not bypassed during NIV, high flow from turbine-driven ventilators can make physiological humidification less effective.
Active humidification devices are preferred in NIV.7 There are two types available for home care ventilators: internal humidifiers (HI; built into the ventilator) and external humidifiers (HE; often connected to the ventilator by passive, unheated tubing). Humidifiers could be beneficial in reducing airflow resistance and patient discomfort and intolerance,4,5 usually linked to NIV failure. Even when NIV fails, humidification turns out to be useful in some studies in facilitating patient intubation when indicated.6 However, only recent studies have examined the effect of using different types of hospital care humidifiers, heaters and tubing on the performance of ventilators regarding pressurization.8 To date, no studies have examined this effect on home care ventilators.
The aim of the present study was to assess the effect on ventilator pressurization performance of adding an external or internal (built-in) humidifier to the patient-ventilator circuit.
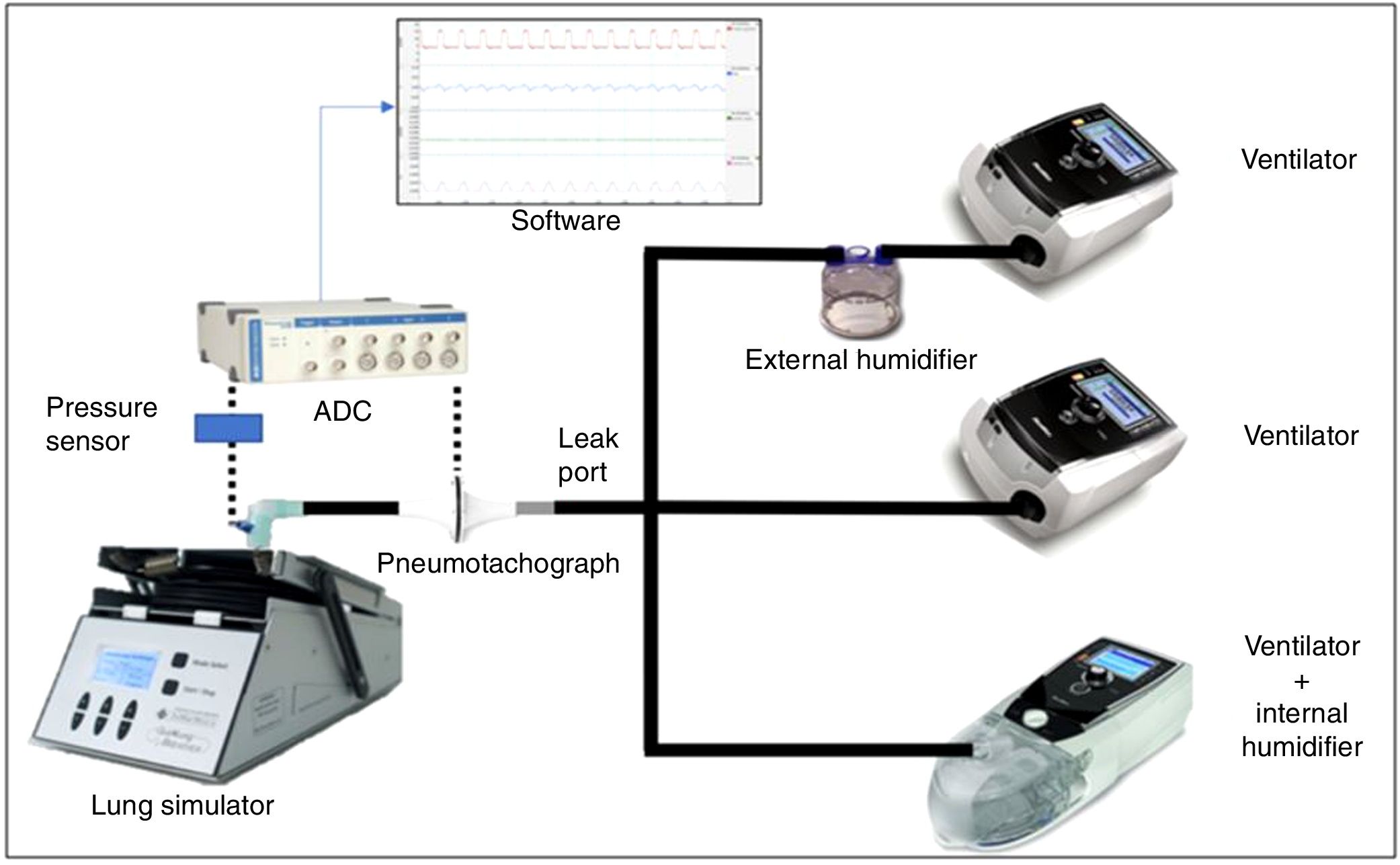
Material and methodsWe conducted an experimental bench study connecting 5 NIV devices commonly employed in home non-invasive ventilation to a lung simulator. The effect on pressurization performance was calculated using the pressure-time product in the first 300 and 500ms under three different clinical conditions with regard to inspiratory effort (no effort-controlled breaths, medium effort and high effort). We evaluated the performance of the ventilator regarding humidification in three different situations: no humidification (ambient air, no humidifier device attached), external humidifier (HE) and internal (built-in) humidifier (HI).
Simulation modelA lung simulator device (QuickLung® + Breather®, Ingmar Medical, Pittsburgh, PA United States) was used for all tests. This device is based on a pneumatic balloon, and it can emulate a wide range of different lung compliance and airway resistance values, also allowing us to set the required respiratory rate, inspiration:expiration (I:E) ratio and spontaneous tidal volume (TV).
Previous studies9 have established the relationship between the level of patient inspiratory effort and the variable P 0.1, defined as the airway pressure generated over 100ms by the inspiratory effort. Thus, a P 0.1 value of −4cmH2O is correlated with medium inspiratory effort, and a P 0.1 value of −8cmH2O is correlated with high inspiratory effort. Taking this into account, we set the simulator device to the required tidal volume to achieve these P 0.1 values, emulating the aforementioned three different scenarios: no effort (controlled ventilation), medium effort (assisted ventilation, simulator tidal volume 350mL) and high effort (assisted ventilation, simulator tidal volume 610mL).
A pre-defined compliance value of 50mL/cmH2O and an airway resistance value of 20cmH2O/L/s was set in the model to simulate a patient with a severe obstructive ventilator disease, as these obstructive patients may be more prone to flow demand and under-assistance when low pressurization rates are employed. We set a spontaneous respiratory rate of 15bpm, with an I:E ratio of 0.45.
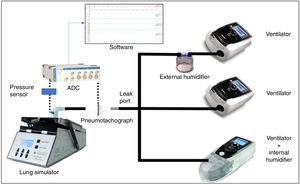
Standard 22-mm diameter and 160-cm length (Model 5805000, Intersurgical®, Berkshire, United Kingdom) tubing was employed to connect the simulator device with each evaluated ventilator. We attached a standard, calibrated passive leak port to the circuit, proximal to the simulator (Whisper Swivel®, Philips Respironics, Murrysville, PA United States), similar to others commonly used in hospital NIV (Fig. 1).
Measurement of variablesThe flow delivered by each ventilator was measured by a calibrated pneumotachograph (MLT1000L®, Ad Instruments, New South Wales, Australia) located close to the simulator inlet distal to the intentional leak. The signal was acquired, filtered and processed through a differential pressure sensor (SpirometryPod®, Ad Instruments, New South Wales, Australia). The pressure was measured by a high-precision sensor, and the signal was conditioned and filtered with a pressure transducer (MLT380® and BridgePod®, Ad Instruments, New South Wales, Australia).
Both flow and pressure signals were digitalized at an acquisition rate of 200Hz with an analogue to digital converter (PowerLab® 26T, Ad Instruments, New South Wales, Australia), and data were subsequently analysed with dedicated software (LabChart® 8 and Peak Analysis, Ad Instruments, New South Wales, Australia) (Fig. 1).
Study protocolWe tested five different home NIV devices commonly employed in our NIV unit and easily available in the EU. All of them were suitable for the use of an internal (built-in) humidifier and an external humidifier:
Ventilator 1: Vivo®40, Breas Medical, Mölnlycke, Sweden.
Ventilator 2: Stellar®150, ResMed, San Diego, CA United States.
Ventilator 3: VPAP S9®, ResMed, San Diego, CA United States.
Ventilator 4: DreamStation®, Philips Respironics, Murrysville, PA United States.
Ventilator 5: Lumis®150, ResMed, San Diego, CA United States.
Each ventilator was programmed with common pre-set parameters: 1. IPAP 15cmH2O, EPAP 4cmH2O; 2. Backup respiratory rate 5bpm; 3. Rise time value as low as allowed by each device; 4. Inspiratory trigger as sensitive as possible without auto-triggering; and 5. Expiratory trigger 50 % of peak inspiratory flow (in this case, ventilator 4 was programmed Autotrak mode). The maximum inspiratory time, when adjustable, was set as high as possible to avoid time-cycling. In devices 1 and 4, a pre-set inspiratory time of 1.2s was set for controlled breaths. Whenever a circuit recognition option was present (ventilators 2 and 4), circuit recognition was performed before each test.
Each ventilator was tested in three different scenarios according to humidifiers: no humidification, external humidifier (HE) and internal “built-in” humidifier (HI) (Fig. 1). The external humidifier employed was an HC500® (Fisher&Paykel®, East Tamaki, New Zealand), commonly recommended by manufacturers. We used room-temperature water (approximately 25°C) to avoid differences in air temperature and density, which could add extra variability to our measurements. All tests were performed the same day within a time frame of 4h and in room air with no added oxygen. A small 22-mm tube connected the humidifier chamber to the ventilator when the HE was used (24-inch gray 1006833 REMstar® humidifier tubing, Philips Respironics, Murrysville, PA, United States)
In this model, the total circuit air volume (so-called compliant volume) resulting after the addition of the humidifier was considerably higher with HE (350mL) than with HI (200mL); both were always filled to the maximum recommended level to reduce compliant volume as much as possible.
With respect to the ventilators, three different humidifier situations and three different efforts were tested, so each ventilator was tested in 9 different model configurations.
Two minutes of digital flow/time and pressure/time (P/T) curves were recorded in each situation, and data from five consecutive representative respiratory cycles (specifically, data concerning five consecutive P/T curves) were selected for analysis in each case. The analysis was carried out using the Peak Analysis tool (LabChart® 8), obtaining the median area under the P/T curve (AUC) value, both in the first 300ms and in the first 500ms of each cycle. These values of PTP300 (cmH2O×ms) and PTP500 (cmH2O×ms) reflect the pressurization rate of the ventilator during the initial phase (PTP300) and during the pressure maintenance phase (PTP500).10
Statistical analysisWe employed SPSS® Statistics v.22 (IBM®, Armonk, NY United States) software for data analysis. Outcome variables (PTP300 and PTP500) were tested for normality with the Kolmogorov-Smirnov and Shapiro-Wilk normality tests. We used the Mann-Whitney U test to contrast the value of PTP300 and PTP500 when each ventilator was connected to HE and HI with its value without a humidification device. The results were considered significant with a p value <0.05.
We performed linear univariate analysis describing the influence of each factor (NIV device, humidification, inspiratory effort) or the distinct combination of factors over the value of PTP300 and PTP500. We considered the F value to be significant when the p value was < 0.05.
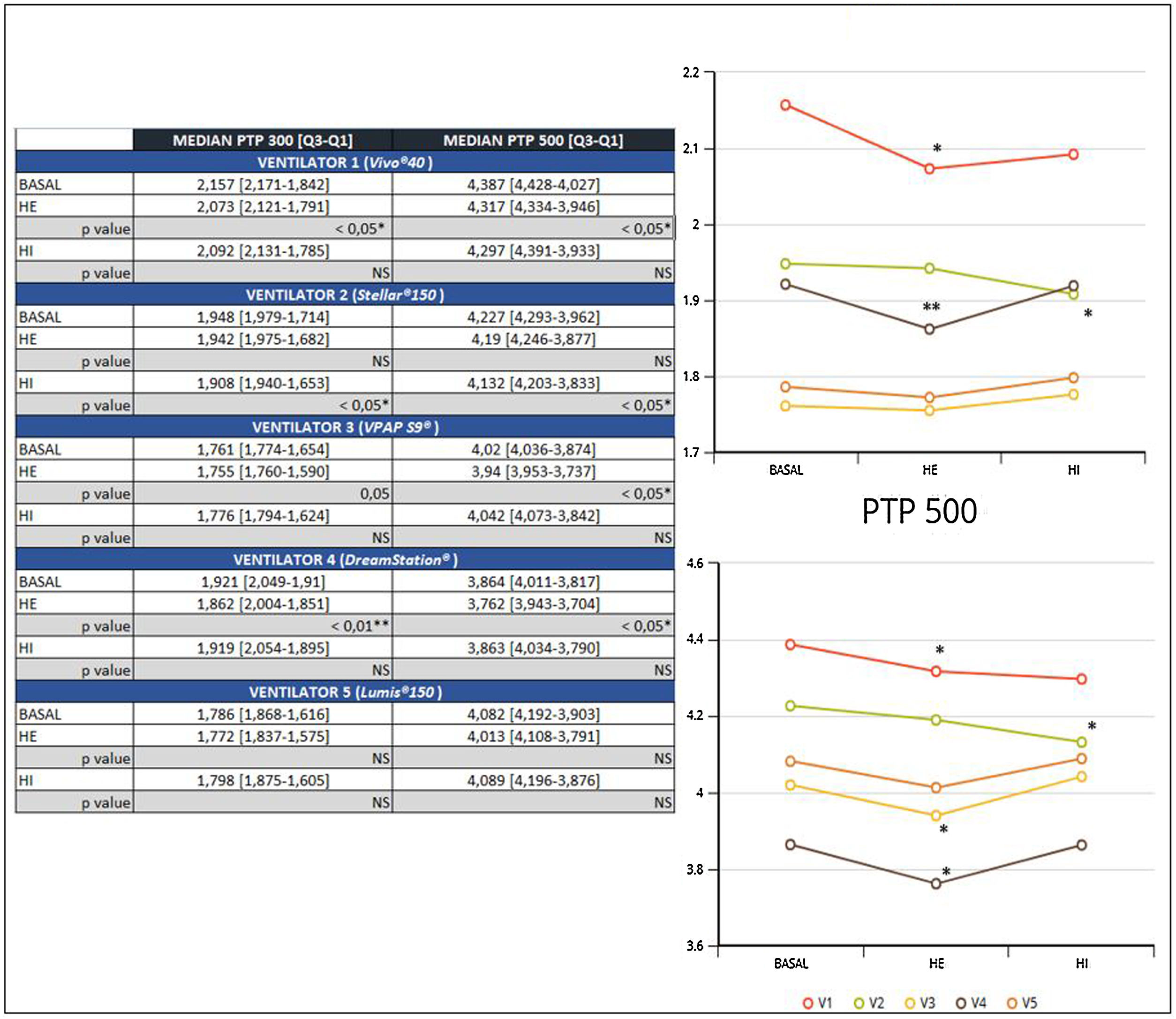
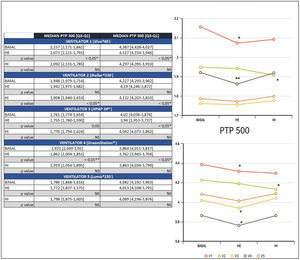
ResultsA significant reduction of PTP300 and PTP500 was found with the use of HE compared with no humidifier in ventilators 1 and 4, and ventilator 3 exhibited a reduction only in PTP500.
In addition, a significant reduction of PTP300 and PTP500 was observed in ventilator 2 with the use of an HI and was not found with an external humidifier.
Ventilator 5 was the only one that could to maintain PTP300 and PTP500, despite the use of different humidifiers and within diverse effort scenarios (Fig. 2).
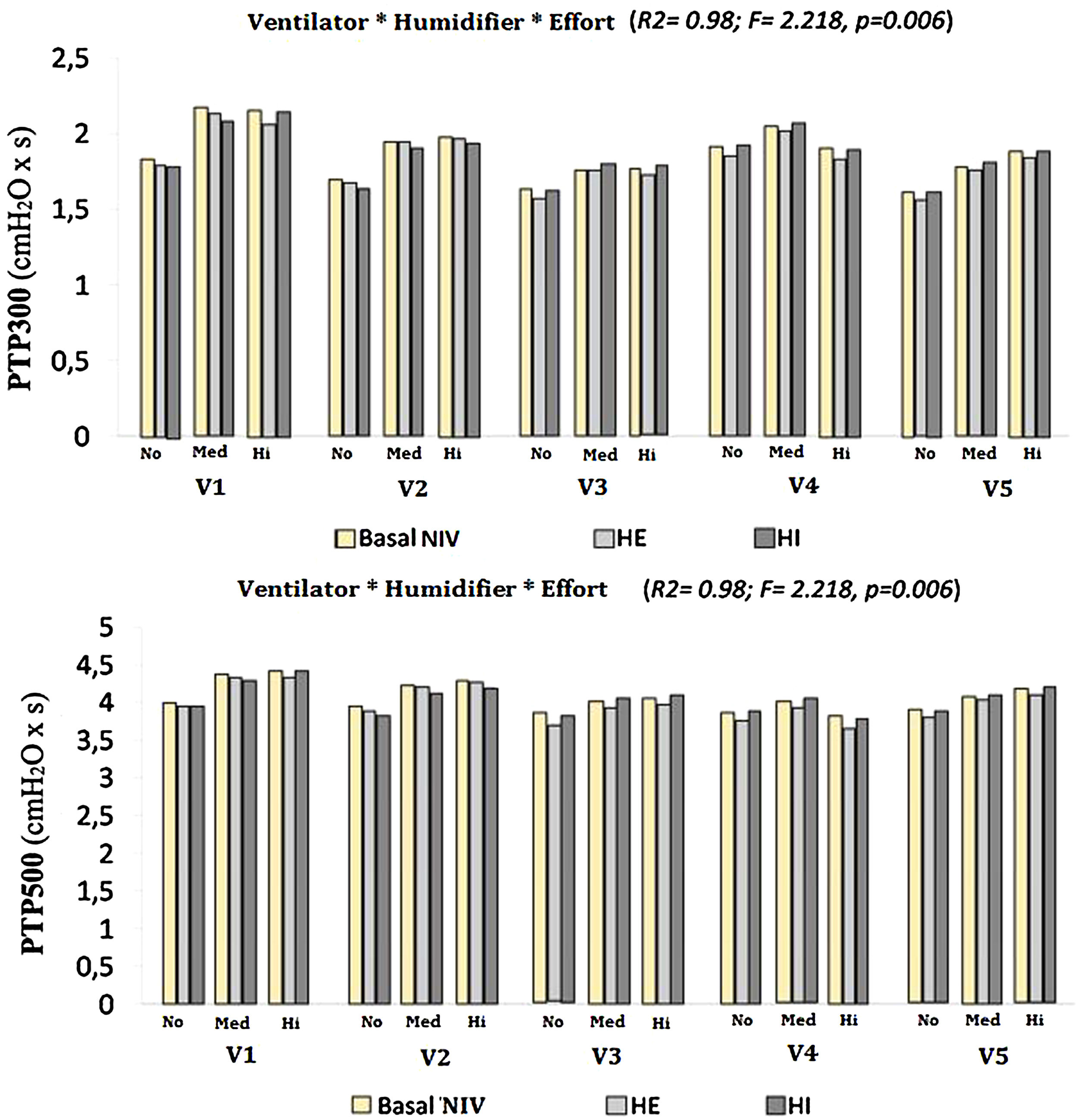
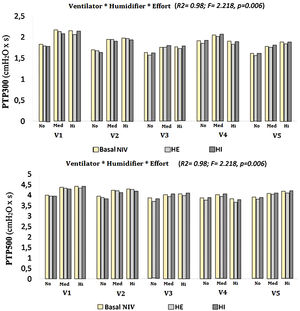
Both the ventilator model and the type of humidification were shown to significantly influence the values of PTP300 and PTP500, up to 46 % and 41 %, respectively (PTP300: F=17.7, p<0.01, R2=0.46; PTP500: F=36.3, p<0.01, R2=0.41). When the degree of inspiratory effort was included, the results were also significant, to the point of increasing the influence over PTP300 and PTP500 to 98 % when the three factors were interacting (F=2.597 and F=2.218, respectively; p<0.01 and R2=0.98 in both cases) (Fig. 3).
A further analysis of the influence of inspiratory effort on each ventilator and humidification scenario was performed. Both PTP300 and PTP500 increased their values with medium and high degrees of effort. Whenever a PTP300 and PTP500 drop occurred with the addition of a humidifier, higher effort led to a greater drop in both variables (Fig. 3).
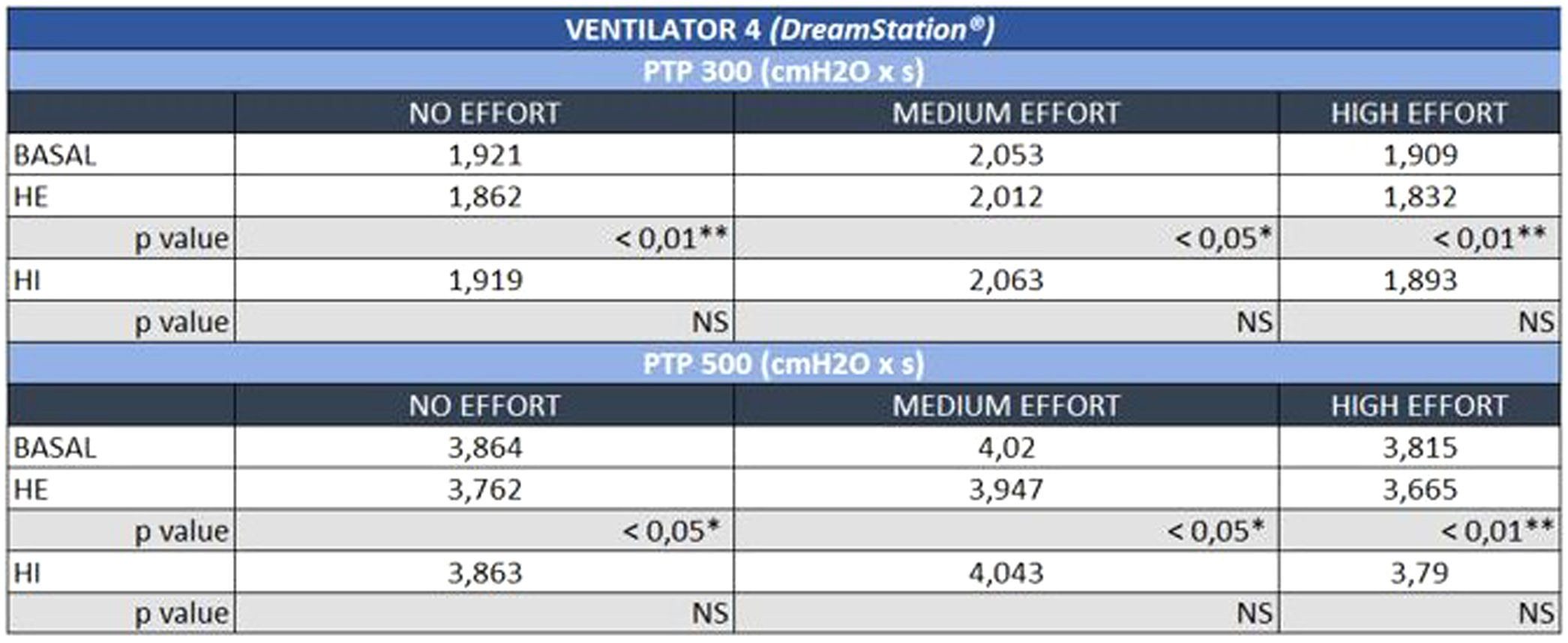
The negative influence of HE on the pressurization rate of ventilators 1 and 4 was significantly greater with high effort (Mann-Whitney U test, p<0.01) (Fig. 4). Similarly, ventilator 3 showed a drop in PTP500 with HE through different levels of effort. In contrast, the addition of HI did not influence PTP300 in these devices regardless of the varying levels of effort (Fig. 4). Ventilator 5 maintained pressurization with any humidifier and effort setting. In contrast to others, ventilator 2 displayed a pressurization drop with its specific HI through all inspiratory effort scenarios (Mann-Whitney U test, p<0.01).
DiscussionIn the present study, three out of five NIV ventilators displayed a negative impact on pressurization when an external humidifier was employed. This effect was attenuated with the use of a built-in humidifier. This can be explained by the increase in circuit compliant volume due to the addition of humidifiers.
The results were remarkably variable on different ventilators. Presumably, ventilators with the best performance on HI were the ones designed by the manufacturer to implement changes in turbine behaviour when their humidifier was detected (i.e., ventilator 5).
A unique case of worse performance with HI was shown on ventilator 2. According to the information provided by the manufacturer, no changes in turbine behaviour are made in this case when the humidifier is plugged. This humidifier chamber is derived from an older model (VPAP IV®, ResMed, San Diego, CA United States) and is not advisable for use in acute care settings or for invasive ventilation with the built-in humidifier attached.
Similar results were observed in the recent publication by Alonso-Iñigo and co-workers,8 which demonstrate that results on ventilatory measurements (i.e., peak inspiratory pressure and flow, Pplat or tidal volume) show significant variation with distinct single-limb heated wire circuits available for clinical use, in spite of ventilator settings and leak compensation algorithms. The explanation they provide for their results relies on the distinct volume, resistance and turbulent flow generated by the circuits.
The reduction of pressurization efficacy observed in several cases with humidifiers seems to be, according to our results, of larger magnitude with high inspiratory effort. This situation may be more relevant to acute or acute-on-chronic respiratory failure when patient respiratory drive is especially high and vigorous inspiratory effort is exerted. Lellouche et al.11 demonstrated how adding a heat and moisture exchange (HME) filter can increase the work of breathing over a heated humidifier chamber, but no comparison was made regarding their impact over NIV alone in a dedicated turbine ventilator.
In contrast, but equally relevant, chronic NIV setting frequently requires the use of humidifiers, as they are meant to improve day-to-day tolerance. Their utilization is wide, and changes in the ventilator model, humidifier chamber and circuit elements are frequent, with greater or lesser knowledge by the attending physician. This type of modification can distort patient-ventilator synchrony in patients already adapted to the ventilator, potentially modifying the tolerance and efficacy of this therapy in the long-term setting.
Non-invasive ventilation success or failure is determined by many elements, such as the cause and severity of respiratory failure, the neurological or haemodynamic state or, relating to NIV, patient tolerance and patient-ventilator synchrony.8,12,13 The latter is in turn influenced by, among other factors, ventilator pressurization efficacy.
There are some limitations concerning our study. First, the NIV devices analysed were limited to our more frequently used models. Newer devices have improved airway circuit resistance/compliance detection, thus minimizing these effects.
Second, as we previously explained and to avoid additional confounding factors, we performed our measurements using room-temperature water, although the heating function of humidifiers is frequently used in clinical practice.
Another limitation was the absence of a test on restrictive pulmonary defects. With a restrictive disease (i.e., neuromuscular disease or kyphoscoliosis), the patient might not have as high of a drive as those suffering an obstructive disease, and the influence of the humidifier might be less significant in this setting. Previous studies have suggested that patients with obstructive defects could be more prone to asynchrony than patients with restrictive defects.14 Moreover, high resistance in the obstructive airways entails a greater challenge for turbine pressurization, and it was our intention to examine the worst-case scenario to prove our hypothesis.
Finally, even though the simulated mechanical conditions could be similar to those of real patients with obstructive ventilatory defects, our study was performed in a controlled and artificial environment. Thus, the results are restricted to the field of simulation and must be interpreted cautiously. Live studies on patients in clinical practice are needed to provide a contrast to these results.
In conclusion, when a humidifier is used with home NIV devices, careful monitoring of possible pressure drops leading to under-assistance should be performed, especially in the acute setting or for end-stage respiratory patients.15 It is advisable for manufacturers to improve pressurization adjustments when built-in humidifiers are attached to ventilators.
Ethical disclosuresThe authors claim that this publication did not involve the use of human subjects and did not imply animal experiments either. The assessment and recommendations made by local ethical committee were taken into account. Since our study was based on a simulation model, use of informed consent was not necessary for its realization.
Conflicts of interestManel Luján declares speaking fees from ResMed® and Philips Respironics®, being an active member of the Breas® Clinical Advisory Board.
Javier Sayas-Catalán declares fees from lectures and teaching activities from ResMed®, Philips Respironics® and Chiesi®.
Ana Hernández-Voth declares fees from Chiesi®.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.