Information on host factors that contribute to false negative and indeterminate results in interferon‐γ release assays (IGRA) are critical to improve the usefulness of these tests in the fight against tuberculosis (TB) epidemics.
The aim of this study was to estimate and compare the sensitivity of an IGRA and the tuberculin skin test (TST), independently and as a combined approach, in patients with TB and to identify risk factors associated with false negative and indeterminate IGRA results.
MethodsRetrospective cohort study of all active TB notifications with an IGRA result (n = 1230), from 2008 to 2015. 68.0 % (n = 727) of these patients had a TST result interpreted using a 5 mm (TST-5 mm) and 10 mm (TST-10 mm) cutoff. Sensitivity was determined for both tests.
Logistic regression analysis was used to evaluate the association of sociodemographic and clinical factors to the risk of false negative or indeterminate IGRA results.
ResultsIGRA, TST-5 mm and TST-10 mm were positive in 82.4 %, 84.5 % and 78.4 % of the patients that performed both tests. When used combined, IGRA/TST-5 mm sensitivity was 91.7 % and IGRA/TST-10 mm sensitivity was 90.6 %. Age≥65 years, alcohol abuse and pulmonary TB were predictive factors for indeterminate results. Inflammatory diseases and pulmonary TB were statistically associated with false negative IGRA results.
ConclusionInflammatory diseases and pulmonary TB were identified as factors for false negative IGRA results. Our results indicate that the use of both tests in a combined approach, especially in specific risk groups of the population, could increase the sensitivity of the screening process and accelerate the achievement of the WHO End TB Strategy goals.
The timely identification of latent tuberculosis infection (LTBI) cases and implementation of preventive treatment can substantially decrease the risk of people developing tuberculosis (TB) disease from a latent infection and, consequently, reduce TB-related morbidity and mortality, reduce transmission, and the burden of the disease.1
The existing screening tests are indirect methods that only provide immunological evidence of host sensitization to Mycobacterium tuberculosis antigens, do not differentiate accurately between LTBI and active TB or provide evidence regarding the stage and potential progression from infection to disease.2
WHO guidelines recommend the use of the tuberculin skin test (TST) and the interferon‐γ release assays (IGRA) as screening tests.
IGRAs measure T-cell release of interferon‐γ in response to stimulation by two strongly immunogenic but highly specific M. tuberculosis complex antigens (ESAT-6 and CFP-10). Two IGRAs are commercially available, QuantiFERON-TB Gold assay (Qiagen) and the T-SPOT.TB assay (Oxford Immunotec), both detecting the release of interferon‐γ in response to the specific M. tuberculosis antigens through different methods.
IGRAs have higher specificity for M. tuberculosis than the TST, as the antigens used are not encoded in the genomes of any of the BCG vaccine strains or most species of nontuberculous mycobacteria.3 Furthermore, these tests have logistic advantages when compared to TST (e.g. require only one visit), have positive and negative controls (e.g. identification of potential cases of anergy), and no boost effect when repeated.2
However, these assays are more expensive and require laboratory infrastructure. Also, T-cell assays such as IGRA are susceptible to variability by different factors at multiple levels, including assay manufacturing, preanalytical processing (e.g. blood volume, sample transport temperature, incubation time) or analytical testing.3
IGRAs have shown reduced sensitivity in immunocompromised patients, especially in those with a severe immune depression, such as patients with diabetes4 or HIV infection.5 IGRAs have also presented a lower sensitivity in young (<5years of age) or immunocompromised children.6,7
Another drawback of IGRA testing is the occurrence of indeterminate results, which may result from an insufficient immune response to the positive control or a high level of response in the negative control,8 with previous studies presenting rates of indeterminate IGRA results ranging between 2 % and 11 %.8
In order to improve the usefulness of the IGRAs as a diagnostic aid for detection of LTBI, it is essential to better understand which factors are associated with false negative and indeterminate IGRA results.
Since there is no diagnostic gold standard for LTBI to help evaluate the IGRAs, sensitivity can be estimated using surrogate reference standards such as IGRA results from patients with a definitive TB diagnosis and, through collected epidemiological data, determine factors that may be associated with false negative results.
The objective of this study was to analyse the performance of an IGRA test in patients with active TB. The specific objectives were to: (i) determine and compare the sensitivity of IGRA and TST tests; (ii) search for risk factors that could be linked with indeterminate IGRA results; and (iii) ascertain risk factors that could be associated with false negative IGRA results.
Material and methodsStudy design and data sourceRetrospective cohort study centered on data from active TB cases notified in the Portuguese National Tuberculosis Surveillance System (SVIG-TB), from 2008 to 2015. This official database is generated by direct compulsory reporting by health care providers and the data from this official system of notification is complemented and updated during TB cases follow-up. Patients notified in the database are diagnosed based on laboratory confirmed TB (through identification of M. tuberculosis by microscopy, cultural and/or molecular methods) and/or based on clinical and radiological findings and a favorable TB treatment response.
For this study ethics committee approval or informed consent was not required, as all patient’s information retrieved from the SVIG-TB database was fully anonymized.
Study dataThe study included all patients notified in the SVIG-TB database with an IGRA test result. The IGRA test used in the patients enrolled in the study was the QuantiFERON-TB Gold In-Tube (Qiagen).
The clinical and sociodemographic variables analyzed were sex, age group, comorbidities (chronic renal failure in dialysis, oncologic diseases, inflammatory diseases, HIV infection, chronic obstructive pulmonary disease [COPD] and diabetes), substance abuse (alcohol and drug abuse) and site of disease (pulmonary or extrapulmonary TB). IGRA and TST results were the dependent variable (event: negative results).
Indeterminate IGRA results were assessed by dividing the results into determinate (positive and negative results) and indeterminate (event).
Statistical analysisTST induration measurements were interpreted using two cutoff points, ≥5 mm (TST-5 mm) and ≥10 mm (TST-10 mm),9 and converted to positive or negative results.
Results from patients that underwent both diagnostic tests were used to calculate the sensitivity with 95 % confidence interval for each test separately and in combination (IGRA/TST-5 mm or IGRA/TST-10 mm). Sensitivity outcomes were compared using McNemar’s test.10
Bivariate and multivariate logistic regression analysis10 was used to determine the variables that were significantly associated with the occurrence of indeterminate and false negative IGRA results. Multivariate logistic regression analysis with forward stepwise (Likelihood Ratio) method, logit function (entry-0.05; removal-0.10), included variables that were significant in bivariate analyses. Odds ratio were reported with 95 % confidence intervals (95 %CI). Statistical analyses were performed using IBM® SPSS® Statistics for Windows, version 23 (IBM Corp., N.Y., USA).
ResultsThere were 1230 patients notified with active TB in the SVIG-TB database (2008–2015) with an IGRA test, 857 patients (69.7 %) had a positive test result, 212 (17.2 %) had a negative result and 161 (13.1 %) had an indeterminate result. Among the 1069 patients with a determinate IGRA result, 727 (68.0 %) also had a TST result.
The mean (±standard deviation) age of the patients with a determinate IGRA result (n = 1069, 86.9 %) was 47.9 (±20.0), ranging from less than 1 to 91 years. Portugal was the main country of origin (n = 931; 87.1 %), followed by Angola (n = 31, 2.9 %), Cabo Verde (n = 23, 2.2 %), Guinea-Bissau (n = 19, 1.8 %) and Mozambique (n = 12, 1.1 %).
Diabetes mellitus was the most common comorbidity and chronic renal failure was the least frequent.
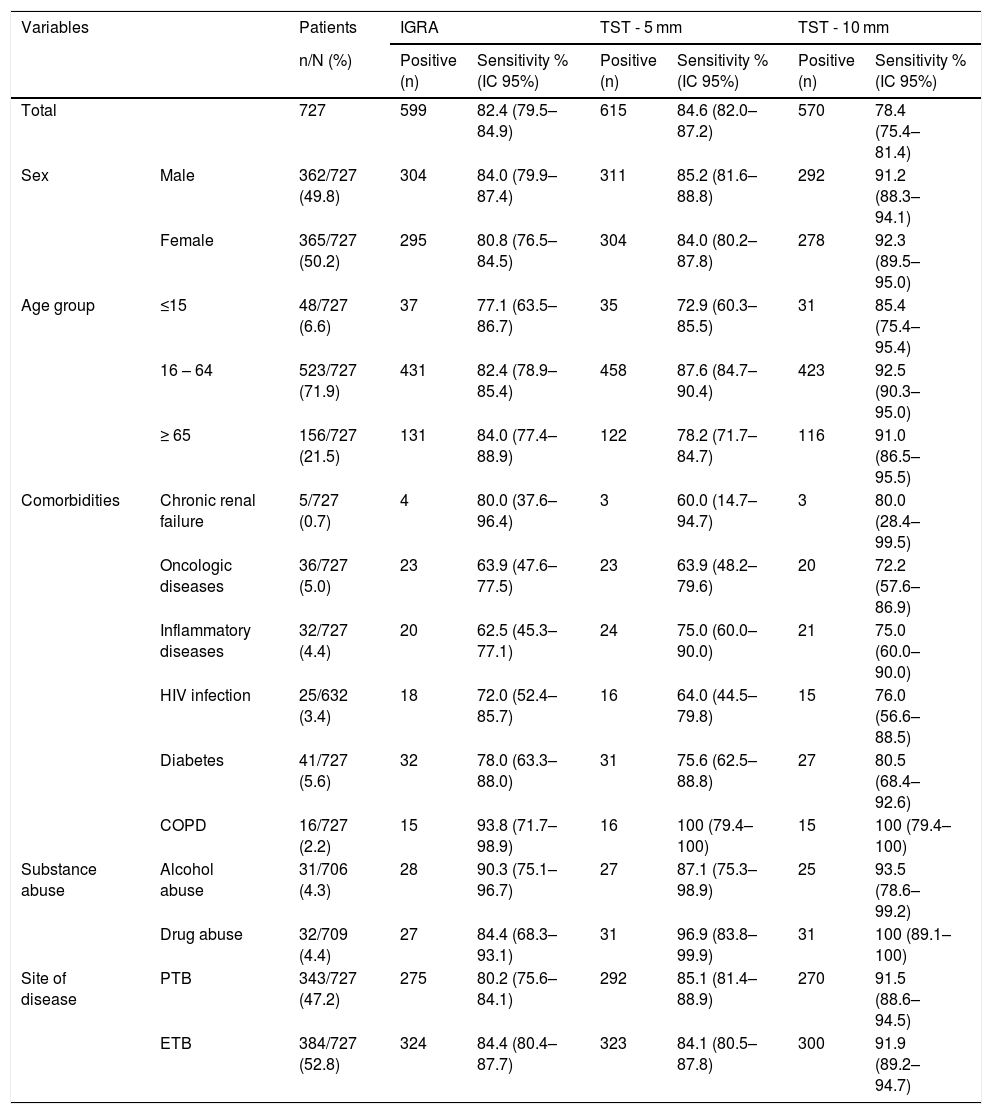
IGRA and TST sensitivityConsidering the patients who performed IGRA and TST assays (n = 727), overall sensitivity was 82.4 %, 84.6 % and 78.4 % for IGRA, TST-5 mm and TST-10 mm, correspondingly. These results indicate that 128 (17.6 %), 112 (15.4 %) and 157 (21.6 %) patients with a diagnosis of active TB had a false negative result with IGRA, TST-5 mm and TST-10 mm tests, respectively. Table 1 presents the sensitivity results for IGRA and TST for total of patients and stratified by sociodemographic and clinical variables.
IGRA and TST sensitivity (5 mm and 10 mm cut-offs) according to sex, age group, comorbidities (chronic renal failure, oncologic disease, inflammatory disease, HIV, diabetes and COPD), substance abuse (alcohol or drug abuse) and site of disease (pulmonary or extrapulmonary) in patients submitted to both assays (n = 727).
| Variables | Patients | IGRA | TST - 5 mm | TST - 10 mm | ||||
|---|---|---|---|---|---|---|---|---|
| n/N (%) | Positive (n) | Sensitivity % (IC 95%) | Positive (n) | Sensitivity % (IC 95%) | Positive (n) | Sensitivity % (IC 95%) | ||
| Total | 727 | 599 | 82.4 (79.5–84.9) | 615 | 84.6 (82.0–87.2) | 570 | 78.4 (75.4–81.4) | |
| Sex | Male | 362/727 (49.8) | 304 | 84.0 (79.9–87.4) | 311 | 85.2 (81.6–88.8) | 292 | 91.2 (88.3–94.1) |
| Female | 365/727 (50.2) | 295 | 80.8 (76.5–84.5) | 304 | 84.0 (80.2–87.8) | 278 | 92.3 (89.5–95.0) | |
| Age group | ≤15 | 48/727 (6.6) | 37 | 77.1 (63.5–86.7) | 35 | 72.9 (60.3–85.5) | 31 | 85.4 (75.4–95.4) |
| 16 – 64 | 523/727 (71.9) | 431 | 82.4 (78.9–85.4) | 458 | 87.6 (84.7–90.4) | 423 | 92.5 (90.3–95.0) | |
| ≥ 65 | 156/727 (21.5) | 131 | 84.0 (77.4–88.9) | 122 | 78.2 (71.7–84.7) | 116 | 91.0 (86.5–95.5) | |
| Comorbidities | Chronic renal failure | 5/727 (0.7) | 4 | 80.0 (37.6–96.4) | 3 | 60.0 (14.7–94.7) | 3 | 80.0 (28.4–99.5) |
| Oncologic diseases | 36/727 (5.0) | 23 | 63.9 (47.6–77.5) | 23 | 63.9 (48.2–79.6) | 20 | 72.2 (57.6–86.9) | |
| Inflammatory diseases | 32/727 (4.4) | 20 | 62.5 (45.3–77.1) | 24 | 75.0 (60.0–90.0) | 21 | 75.0 (60.0–90.0) | |
| HIV infection | 25/632 (3.4) | 18 | 72.0 (52.4–85.7) | 16 | 64.0 (44.5–79.8) | 15 | 76.0 (56.6–88.5) | |
| Diabetes | 41/727 (5.6) | 32 | 78.0 (63.3–88.0) | 31 | 75.6 (62.5–88.8) | 27 | 80.5 (68.4–92.6) | |
| COPD | 16/727 (2.2) | 15 | 93.8 (71.7–98.9) | 16 | 100 (79.4–100) | 15 | 100 (79.4–100) | |
| Substance abuse | Alcohol abuse | 31/706 (4.3) | 28 | 90.3 (75.1–96.7) | 27 | 87.1 (75.3–98.9) | 25 | 93.5 (78.6–99.2) |
| Drug abuse | 32/709 (4.4) | 27 | 84.4 (68.3–93.1) | 31 | 96.9 (83.8–99.9) | 31 | 100 (89.1–100) | |
| Site of disease | PTB | 343/727 (47.2) | 275 | 80.2 (75.6–84.1) | 292 | 85.1 (81.4–88.9) | 270 | 91.5 (88.6–94.5) |
| ETB | 384/727 (52.8) | 324 | 84.4 (80.4–87.7) | 323 | 84.1 (80.5–87.8) | 300 | 91.9 (89.2–94.7) | |
PTB = Pulmonary tuberculosis; ETB = Extrapulmonary tuberculosis.
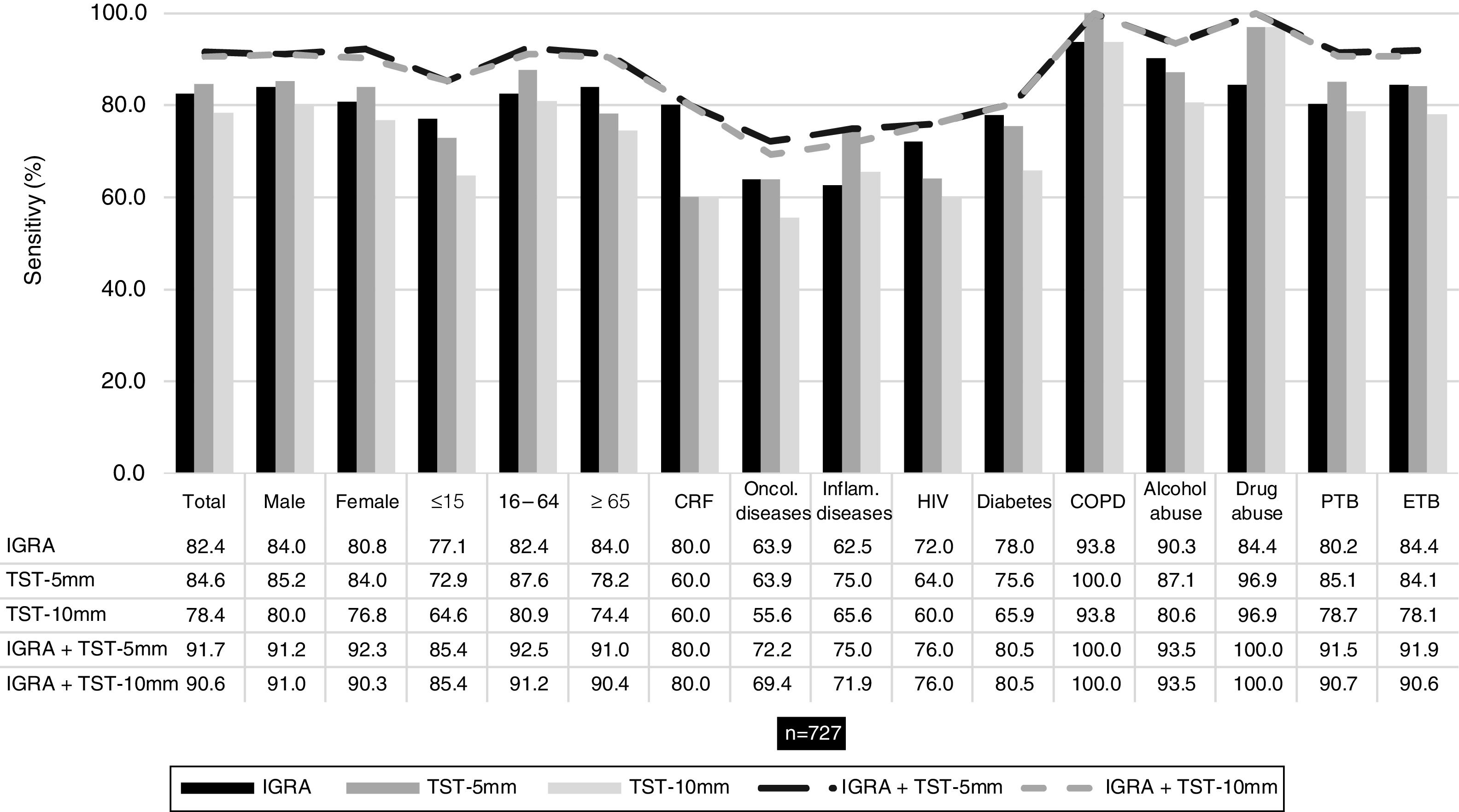
Comparing the sensitivity of the tests separately with the combined sensitivity of IGRA/TST-5 mm and IGRA/TST-10 mm (91.7 % and 90.6 %, respectively), the latter was consistently higher and this difference was statistically significant (p < 0.001) – Fig. 1.
Sensitivity of IGRA and TST when used separately and when used together (IGRA/TST-5 mm and IGRA/TST-10 mm) in patients with both test results (n = 727). Sensitivity was assessed according to sex, age group (≤15, 16-64, ≥65 years), comorbidities (chronic renal failure [CRF], oncologic disease, inflammatory disease, HIV infection, diabetes and COPD), substance abuse (alcohol or drug abuse) and site of disease (pulmonary TB [PTB] or extrapulmonary TB [ETB]).
Of the 161 patients with an indeterminate result, the majority were male (67.7 %) with a mean age of 56.0 (±19.4) that ranged from 5 to 92 years, and more than half were ≥50 years old (57.1 %).
The proportion of indeterminate results increases as the age increases, with patients over 80 years old presenting the highest proportion of indeterminate results (34.5 %) and patients under 20 years old presenting the lowest proportion of indeterminate results (2.9 %).
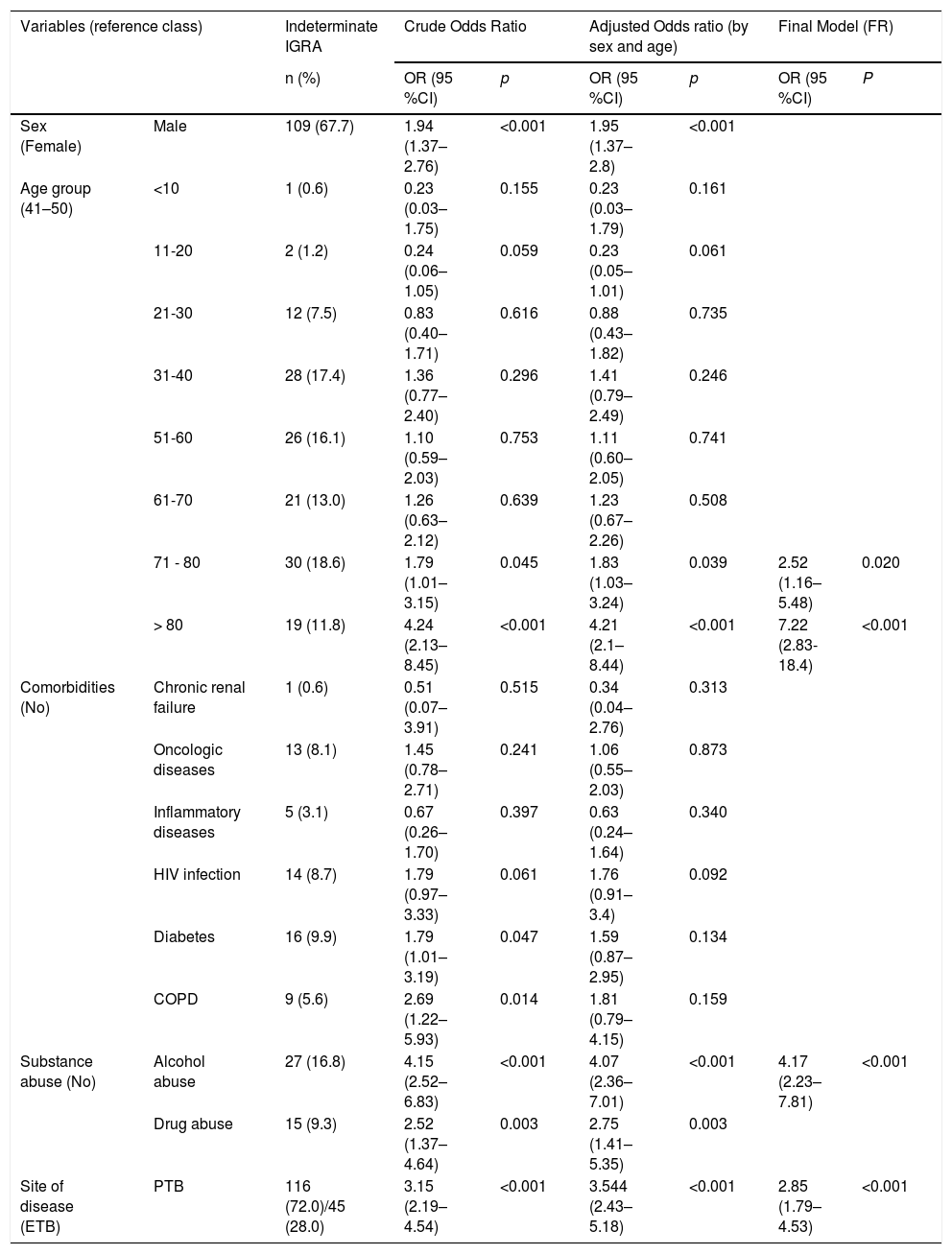
Being older than 70 years, alcohol abuse and pulmonary TB were independent predictive factors with a statistically significant association to higher probability of indeterminate IGRA results in TB patients (Table 2).
Sociodemographic and clinical characteristics of 161 patients with indeterminate IGRA results and their association with the occurrence of the indeterminate test results in patients with active TB.
| Variables (reference class) | Indeterminate IGRA | Crude Odds Ratio | Adjusted Odds ratio (by sex and age) | Final Model (FR) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | OR (95 %CI) | p | OR (95 %CI) | p | OR (95 %CI) | P | ||
| Sex (Female) | Male | 109 (67.7) | 1.94 (1.37–2.76) | <0.001 | 1.95 (1.37–2.8) | <0.001 | ||
| Age group (41–50) | <10 | 1 (0.6) | 0.23 (0.03–1.75) | 0.155 | 0.23 (0.03–1.79) | 0.161 | ||
| 11-20 | 2 (1.2) | 0.24 (0.06–1.05) | 0.059 | 0.23 (0.05–1.01) | 0.061 | |||
| 21-30 | 12 (7.5) | 0.83 (0.40–1.71) | 0.616 | 0.88 (0.43–1.82) | 0.735 | |||
| 31-40 | 28 (17.4) | 1.36 (0.77–2.40) | 0.296 | 1.41 (0.79–2.49) | 0.246 | |||
| 51-60 | 26 (16.1) | 1.10 (0.59–2.03) | 0.753 | 1.11 (0.60–2.05) | 0.741 | |||
| 61-70 | 21 (13.0) | 1.26 (0.63–2.12) | 0.639 | 1.23 (0.67–2.26) | 0.508 | |||
| 71 - 80 | 30 (18.6) | 1.79 (1.01–3.15) | 0.045 | 1.83 (1.03–3.24) | 0.039 | 2.52 (1.16–5.48) | 0.020 | |
| > 80 | 19 (11.8) | 4.24 (2.13–8.45) | <0.001 | 4.21 (2.1–8.44) | <0.001 | 7.22 (2.83-18.4) | <0.001 | |
| Comorbidities (No) | Chronic renal failure | 1 (0.6) | 0.51 (0.07–3.91) | 0.515 | 0.34 (0.04–2.76) | 0.313 | ||
| Oncologic diseases | 13 (8.1) | 1.45 (0.78–2.71) | 0.241 | 1.06 (0.55–2.03) | 0.873 | |||
| Inflammatory diseases | 5 (3.1) | 0.67 (0.26–1.70) | 0.397 | 0.63 (0.24–1.64) | 0.340 | |||
| HIV infection | 14 (8.7) | 1.79 (0.97–3.33) | 0.061 | 1.76 (0.91–3.4) | 0.092 | |||
| Diabetes | 16 (9.9) | 1.79 (1.01–3.19) | 0.047 | 1.59 (0.87–2.95) | 0.134 | |||
| COPD | 9 (5.6) | 2.69 (1.22–5.93) | 0.014 | 1.81 (0.79–4.15) | 0.159 | |||
| Substance abuse (No) | Alcohol abuse | 27 (16.8) | 4.15 (2.52–6.83) | <0.001 | 4.07 (2.36–7.01) | <0.001 | 4.17 (2.23–7.81) | <0.001 |
| Drug abuse | 15 (9.3) | 2.52 (1.37–4.64) | 0.003 | 2.75 (1.41–5.35) | 0.003 | |||
| Site of disease (ETB) | PTB | 116 (72.0)/45 (28.0) | 3.15 (2.19–4.54) | <0.001 | 3.544 (2.43–5.18) | <0.001 | 2.85 (1.79–4.53) | <0.001 |
OR = Odds ratio; CI = confidence interval; PTB = Pulmonary tuberculosis; ETB = Extrapulmonary tuberculosis. FR - Forward stepwise (LR).
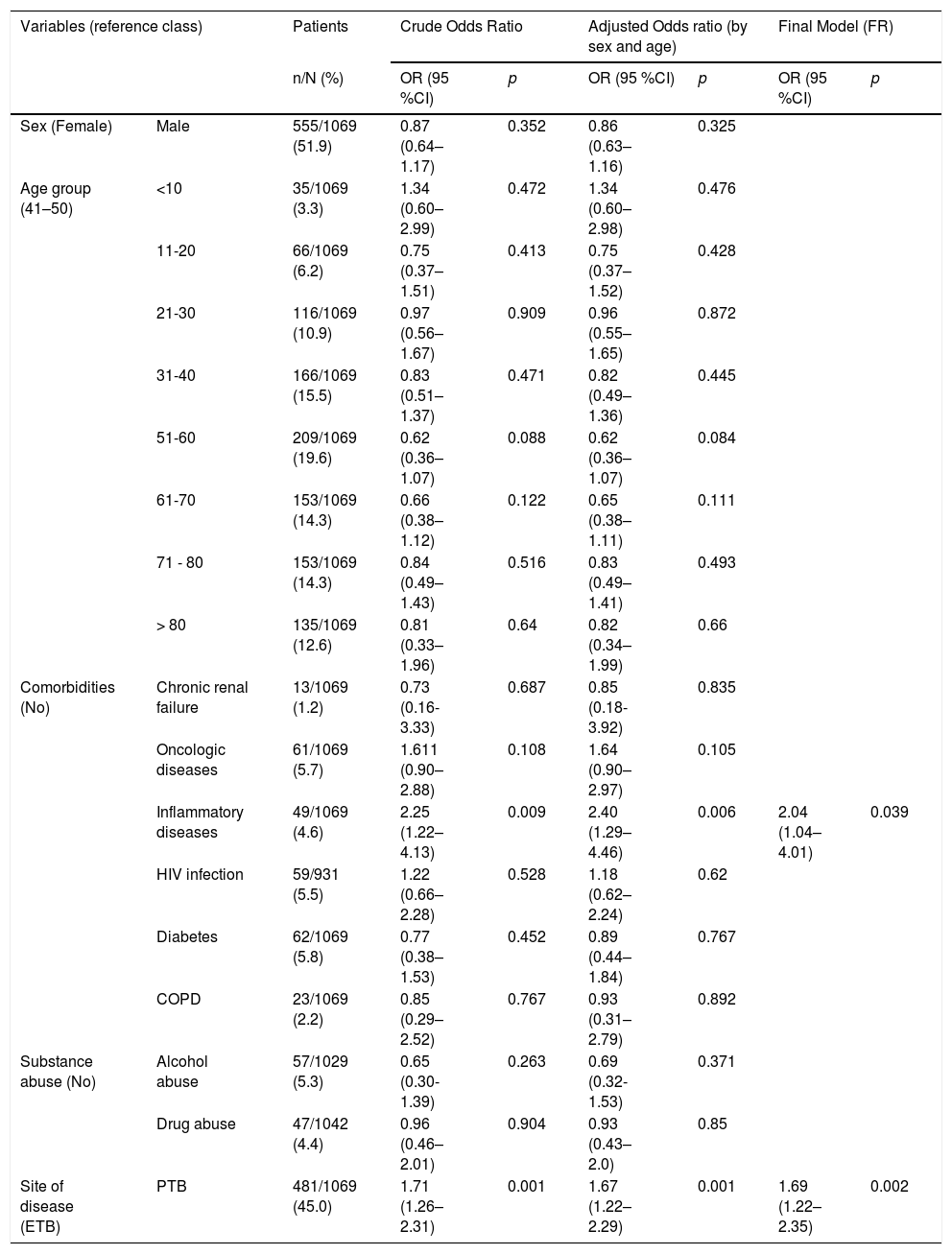
Among the risk factors analyzed through logistic regression (Table 3), two presented a statistically significant association with an increased risk of obtaining false negative IGRA results - inflammatory diseases and pulmonary TB.
Association between false negative IGRA results and sociodemographic and clinical variables among patients with notified TB (n = 1069).
| Variables (reference class) | Patients | Crude Odds Ratio | Adjusted Odds ratio (by sex and age) | Final Model (FR) | ||||
|---|---|---|---|---|---|---|---|---|
| n/N (%) | OR (95 %CI) | p | OR (95 %CI) | p | OR (95 %CI) | p | ||
| Sex (Female) | Male | 555/1069 (51.9) | 0.87 (0.64–1.17) | 0.352 | 0.86 (0.63–1.16) | 0.325 | ||
| Age group (41–50) | <10 | 35/1069 (3.3) | 1.34 (0.60–2.99) | 0.472 | 1.34 (0.60–2.98) | 0.476 | ||
| 11-20 | 66/1069 (6.2) | 0.75 (0.37–1.51) | 0.413 | 0.75 (0.37–1.52) | 0.428 | |||
| 21-30 | 116/1069 (10.9) | 0.97 (0.56–1.67) | 0.909 | 0.96 (0.55–1.65) | 0.872 | |||
| 31-40 | 166/1069 (15.5) | 0.83 (0.51–1.37) | 0.471 | 0.82 (0.49–1.36) | 0.445 | |||
| 51-60 | 209/1069 (19.6) | 0.62 (0.36–1.07) | 0.088 | 0.62 (0.36–1.07) | 0.084 | |||
| 61-70 | 153/1069 (14.3) | 0.66 (0.38–1.12) | 0.122 | 0.65 (0.38–1.11) | 0.111 | |||
| 71 - 80 | 153/1069 (14.3) | 0.84 (0.49–1.43) | 0.516 | 0.83 (0.49–1.41) | 0.493 | |||
| > 80 | 135/1069 (12.6) | 0.81 (0.33–1.96) | 0.64 | 0.82 (0.34–1.99) | 0.66 | |||
| Comorbidities (No) | Chronic renal failure | 13/1069 (1.2) | 0.73 (0.16-3.33) | 0.687 | 0.85 (0.18-3.92) | 0.835 | ||
| Oncologic diseases | 61/1069 (5.7) | 1.611 (0.90–2.88) | 0.108 | 1.64 (0.90–2.97) | 0.105 | |||
| Inflammatory diseases | 49/1069 (4.6) | 2.25 (1.22–4.13) | 0.009 | 2.40 (1.29–4.46) | 0.006 | 2.04 (1.04–4.01) | 0.039 | |
| HIV infection | 59/931 (5.5) | 1.22 (0.66–2.28) | 0.528 | 1.18 (0.62–2.24) | 0.62 | |||
| Diabetes | 62/1069 (5.8) | 0.77 (0.38–1.53) | 0.452 | 0.89 (0.44–1.84) | 0.767 | |||
| COPD | 23/1069 (2.2) | 0.85 (0.29–2.52) | 0.767 | 0.93 (0.31–2.79) | 0.892 | |||
| Substance abuse (No) | Alcohol abuse | 57/1029 (5.3) | 0.65 (0.30-1.39) | 0.263 | 0.69 (0.32-1.53) | 0.371 | ||
| Drug abuse | 47/1042 (4.4) | 0.96 (0.46–2.01) | 0.904 | 0.93 (0.43–2.0) | 0.85 | |||
| Site of disease (ETB) | PTB | 481/1069 (45.0) | 1.71 (1.26–2.31) | 0.001 | 1.67 (1.22–2.29) | 0.001 | 1.69 (1.22–2.35) | 0.002 |
OR = Odds ratio; CI = confidence interval; PTB = Pulmonary tuberculosis; ETB = Extrapulmonary tuberculosis.
The objective of this study was to analyse the performance of an IGRA test in patients with active TB which involved establishing and comparing the sensitivity of IGRA and TST tests.
IGRA sensitivity results are heterogeneous across the literature, ranging from 60 %11 to nearly 100 %12 with most studies presenting an sensitivity above 70 %, as demonstrated by three different meta-analyzes that obtained a pooled sensitivity of 81.0 %,13 80.0 %14 and 84.2 %.15 In our study, IGRA overall sensitivity (82.4 %) was close to the pooled sensitivity of these meta-analyzes. Nonetheless, 17.6 % of the results were false negative, demonstrating that although these tests can facilitate diagnostic decisions, negative results should not be used alone to exclude an M. tuberculosis infection but interpreted in conjunction with other clinical findings and diagnoses.
Our TST sensitivity was higher than the pooled sensitivity obtained in three different meta-analysis - 66 %,15 70 %13 and 77 %16 - but comparable to a US TB surveillance study that included almost 65 000 culture-confirmed TB patients, in which 84.2 % of the patients had a TST≥5 mm and 81.6 % a TST ≥ 10 mm.17 Despite the differences in sensitivity obtained between IGRA and TST-5 mm or TST-10 mm, only the difference between IGRA and TST-10 mm was statistically significant (p = 0.021), which indicates that IGRA and TST-5 mm had an similar performance in the identification of cases of infection and presented a better performance than TST-10 mm.
By combining the results of the two tests, the sensitivity increased to more than 90.0 %, and the difference between these results and those obtained by the tests separately was statistically significant (p < 0.001). Choi et al. presented similar sensitivity results, obtaining 85.3 % (TST), 70.3 % (IGRA) and 93.3 % (IGRA/TST).4 This increase in sensitivity suggests that the combined use of the two tests could promote the identification of more cases of infection than if used separately and in substitution of one another. The combined use could enhance the detection of LTBI cases among vulnerable populations,18 such as homeless people, drug or alcohol abusers, prisoners and people living with HIV. This approach could be especially helpful in low-incidence countries, as the majority of TB cases occur due to the progression from LTBI to TB disease.19 Furthermore, these vulnerable populations are frequently associated with individual and contextual factors, as presented by Zão et al,20 that contribute to patient and healthcare system delay in the diagnosis and treatment of TB. A false negative result could contribute to longer delays, thus perpetuating the spread of the disease.
Indeterminate results represent a considerable problem for clinical management, since they imply the lack of clear information about the patient's TB infection status.
Unlike other studies,21,22 our results do not show old age as a risk factor for false negative IGRA results. However, we observed that older age was a predictive factor for indeterminate results, which was consistent with the findings of other studies.21,23 Several studies analyzed the association between age and indeterminate IGRA results and concluded that indeterminate results are significantly more common in children23–25 and adolescents23 and in the elderly.23,26 Our results confirm part of their conclusions, with patients aged >80 years being seven times more likely to have an indeterminate IGRA result. In addition, when we stratified patients in ten-year age groups, we observed that the proportion of indeterminate results increased as the age increases, with patients over 80 years old presenting the highest proportion of indeterminate results, results shared with Kobashi et al.26 In our study younger patients did not present any association with false negative or indeterminate IGRA results.
Our study also found an association between alcohol abuse and indeterminate IGRA results in which patients with an alcohol problem were four times more likely to have an indeterminate outcome. Both acute and chronic alcohol use have profound regulatory effects on the immune system,27 which possibly impairs the patient’s ability to respond correctly to the IGRA, resulting in an indeterminate result. Because alcohol dependence was based on self-report and daily alcohol intake was not determined, further studies with a more detailed information on alcohol consumption are needed to better understand this potential association.
From the studies available involving IGRA, PTB patients and ETB patients, the test sensitivity remains similar in both forms of TB, as shown by Di et al.28 (89.7 % vs 79.7 %), Ji et al.29 (89.7 % vs 87.6 %), Azghay et al.30 (78.9 % vs 87.8 %) and in our study (80.2 % vs 84.4 %). However, pulmonary TB was presented as a predictive factor for indeterminate and false negative results, in which these patients were almost three times more likely to have an indeterminate outcome and nearly twice as likely to have a false negative result compared to extrapulmonary TB (ETB) patients.
Clinical manifestations of ETB may vary from an acute or disseminated disease, such as meningitis or miliary TB, to a chronic localized infection with an insidious onset and slow progression, such as lymph node or osteoarticular TB.31,32 In two analyses of risk factors of false-negative IGRA results, only meningitis TB from the eight forms of ETB in one study and 6 forms in the other was significantly associated with false negative results, an acute clinical manifestation.28,33 Thus, the fact that most ETB manifestations have a slower progression to severe disease, possibly allows patients to maintain a functional immune system and consequently functional T-cells which, when presented with TB antigens, will respond with the production of interferon‐γ. Another reason for these results may lie in the severity of pulmonary TB cases. Different studies have shown that severe pulmonary disease can be associated with immune suppression which may lead to reduced interferon‐γ production.34,35 During TB progression, the natural cytokine balance is altered while the bacterial load increases, potentially influencing the performance of IGRA tests resulting in a reduction in M. tuberculosis specific immune responses, especially interferon‐γ production.36,37
Considering the existence of highly heterogeneous clinical manifestations associated to the fact that most forms of ETB do not contribute to the transmission of TB, and also considering that research in ETB and their influence in the immune response is scarce, it is imperative to develop further studies in order to better understand the performance of the test in the different clinical manifestations of TB.
We observed that inflammatory diseases were an independent predictive factor for negative IGRA results in patients with active TB. With the growing use of biologic therapies for inflammatory rheumatic diseases and the increased risk of TB associated38 with their use, our results show that the use of IGRA alone may not be sufficient to eliminate the possibility of an M. tuberculosis infection. In view of the high risk of TB in these patients and the possibility of false negative results, a dual testing strategy of IGRA and TST could be more effective for LTBI diagnosis, as other studies involving patients with inflammatory diseases concluded.39–41
In our study, five of the 49 patients with inflammatory diseases presented an indeterminate IGRA result but no significant association was found. Some studies have identified an association,39,42,43 with the corticosteroids used in the treatment of these diseases being the common predictive factor for indeterminate results. No treatment data was available in our study.
Our study has limitations. T-cell assays like IGRA are susceptible to variability by different factors associated with assay manufacturing, pre-analytical processing or analytical testing.3 However, despite their importance we were unable to determine the impact of these factors on the IGRA results used in the study. Due to the absence of healthy individuals in our study population, it was not possible to estimate the specificity for both immunologic tests, TST and IGRA. The presence of variables based on patient self-report (e.g. alcohol abuse, drug abuse) may have led to a less accurate estimate of the impact of these variables. The use of active TB patients as a proxy to assess the sensitivity of IGRA and TST has the disadvantage that patients with active TB may present immunosuppression due to the disease itself, thus obtaining a different response than with patients with a latent infection or recent infection.
On the other hand, our analysis of the performance of the IGRA test was comprehensive. It involved a considerable number of patients with different risk factors, which allowed observation of the performance of IGRA in different contexts, contributing to a better understanding of how to use this test in populations with certain risk factors. In addition, the comparison with the other screening method for LTBI available (TST) provided additional information on how these tests can be used in order to enhance the detection of cases of infection.
ConclusionIn our study we identified three factors associated with the occurrence of indetermined IGRA results - age over 70 years, alcohol abuse and pulmonary TB.
Inflammatory diseases and pulmonary TB were independent risk factors for false negative IGRA results in patients with active TB.
Given the impact that treatments of inflammatory diseases have on the development of active TB, our results suggest a careful interpretation of a negative result, since there is a significant risk of false negative results.
The fact that the IGRA appears to perform well in patients with ETB (less indeterminate and false negative results than in patients with pulmonary TB), makes it a valuable diagnostic tool in these cases.
FundingFundação para a Ciência e Tecnologia provided financial support to conduct this research [Grant: PTDC/SAU-PUB/31346/2017] but had no involvement in the research itself.
Declarations of interestNone.



![Sensitivity of IGRA and TST when used separately and when used together (IGRA/TST-5 mm and IGRA/TST-10 mm) in patients with both test results (n = 727). Sensitivity was assessed according to sex, age group (≤15, 16-64, ≥65 years), comorbidities (chronic renal failure [CRF], oncologic disease, inflammatory disease, HIV infection, diabetes and COPD), substance abuse (alcohol or drug abuse) and site of disease (pulmonary TB [PTB] or extrapulmonary TB [ETB]). Sensitivity of IGRA and TST when used separately and when used together (IGRA/TST-5 mm and IGRA/TST-10 mm) in patients with both test results (n = 727). Sensitivity was assessed according to sex, age group (≤15, 16-64, ≥65 years), comorbidities (chronic renal failure [CRF], oncologic disease, inflammatory disease, HIV infection, diabetes and COPD), substance abuse (alcohol or drug abuse) and site of disease (pulmonary TB [PTB] or extrapulmonary TB [ETB]).](https://static.elsevier.es/multimedia/25310437/0000002600000006/v1_202011020636/S2531043719302090/v1_202011020636/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)




