Nivolumab is an immune checkpoint inhibitor (ICI) agent that targets programmed death receptor-1 (PD-1).1 Immune-related adverse events (irAEs) under treatment with anti-PD-1/anti-PD-L1 antibodies are frequent (relative frequency – 70%).1 For nivolumab, any treatment-related adverse events was documented in 74%–85% of patients, with 12%–20% being grade 3 and 4.2 Encephalitis is an extremely rare and potentially fatal immune-mediated neurological adverse event (nAE).1
A 70-year-old Caucasian male presented a 4-day history of fever, somnolence and vomiting. Past medical history was significant for squamous cell carcinoma of the lung (diagnosed in 2018), initially staged as cT4N0M0–IIB (with chest wall invasion and left pulmonary artery involvement), with PD-L1 value of 0%, and proposed for chemotherapy followed by radical radiotherapy. After four cycles of carboplatin and oral vinorelbine with stable disease, radical radiotherapy was started but not completed due to recurrent infections of the pulmonary mass which resulted in mass enlargement and cavitation. Eastern Cooperative Oncology Group (ECOG) performance status was 1 and second line Nivolumab was started in March 2019, achieving stable disease as best response. At admission, the patient had received a total of 22 doses of Nivolumab (240 mg, every 2 weeks); denied other symptoms and no seizure activity or movement disorders were observed. Patient had no previous history of neurological disorders. Physical examination revealed fever, fluctuation in levels of consciousness, global aphasia and paratonia. Later, predominantly axial rigidity developed. Two days earlier to this admission he had been observed due to behavior alteration, confusion and a fall. A brain CT was performed showing no significant findings.
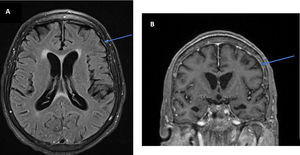
Initial workup revealed only mild anemia and hyponatremia. A brain magnetic resonance imaging (MRI) was performed with and without gadolinium, showing mild meningeal enhancement but no parenchymal alterations suggesting autoimmune or infectious cause (Fig. 1). Analysis of the cerebrospinal fluid (CSF) revealed normal glucose, mild pleocytosis (22/microL) with mononuclear cell predominance and elevated protein levels (113 mg/dL).
Empiric acyclovir and ampicillin were started until exclusion of infectious cause. Given the high suspicion of autoimmune encephalitis, high-dose intravenous methylprednisolone 1 g/d was started and maintained for 5 days, followed by intravenous immunoglobulin therapy 0.4 g/kg/daily for 5 additional days.
After day 1 of methylprednisolone, a major symptomatic improvement was observed, with partial cognitive recovery and apyrexia.
Pending tests from CSF analysis were negative for infectious agents and cytology showed no malignant cells. Cell-surface and intracellular anti-neuronal antibodies were also negative.
During hospitalization, tapering of corticosteroids was performed with continuous improvement in cognition and autonomy. Ultimately, patient was discharged after 16 days of hospitalization medicated with 20 mg of prednisolone. Steroids were tapered and stopped in the subsequent month, with no clinical worsening.
After exclusion of infection, metastatic etiology or other metabolic/endocrine etiologies, our report displays a rare and severe case of central nervous system (CNS) irAE, an immune-related Encephalitis (grade 4). Neurological toxicity occurs in 6.1% of patients receiving PD-1 inhibitors2 and ICI-induced encephalitis was reported in 1% to 3% of cases.3 Vogrig A. et al. characterized CNS complications of ICIs in three clinical phenotypes, limbic encephalitis, cerebellitis and meningoencephalitis, each with distinct immunological background, disease course and treatment response – our report presents a case of meningoencephalitis.4
To the best of our knowledge, only four case reports were found in literature describing association between PD-1 inhibitor nivolumab and autoimmune encephalitis in patients with NSCLC.3,5,6
These events are of concern with a high rate of residual symptoms and even fatal outcomes.1 irEncephalitis mortality rate is as high as 19%.7 Given the increasing use of ICI's, in monotherapy or as combination therapy, the prevalence of irEncephalitis is expected to increase.7
CNS irAEs are rare and knowledge on how to diagnose and treat them is limited.4 Diagnosis is challenging, due to lack of specificity of CNS symptoms,7 wide range of clinical features, lack of diagnostic biomarkers and the extensive list of differential diagnosis.
Brain MRI might be normal, reveal T2/FLAIR hyperintensities with no specific location, gadolinium enhanced or not, findings suggestive of an immune process, such as demyelinating lesions or limbic encephalitis. Our patient had no remarkable changes (Fig. 1).
CSF analysis can also present normal results.7 Anti-Ma2 anti-bodies are usually associated with irEncephalitis,7 and Epstein-Barr virus PCR was identified across multiple CSF samples, suggesting an association between viral infection and irEncephalitis.4 These associations were not found in our case.
Average time of onset of nAEs is 6 weeks to 3 months.4,5 In our case, nAEs appeared 11 months after starting Nivolumab, which stresses the need to remain alert at all phases of treatment with ICI, even after its discontinuation.2
Although causality cannot be proven in these cases, several features suggest that encephalitis was triggered by immune checkpoint blockade and is reversible after prompt cessation of immunotherapy and treatment with high doses of steroid with or without intravenous immunoglobulin therapy.1
Early recognition and successful management of irAEs are key to reducing its morbimortality; multidisciplinary discussion plays an essential role and more relevant cases should be collected and studied.