Pulmonary vein stenosis (PVS) is a rare condition, often difficult to diagnose and associated with poor prognosis at advanced stages. Lung parenchymal abnormalities are indirect evidence of PVS and can manifest as multifocal opacities, nodular lesions, unilateral effusions, and interstitial septal thickening. These can lead to erroneous diagnoses of airway disease, pneumonia, malignancies or interstitial lung disease. This review summarizes the current literature about the approach to, evaluation and management of these patients. Our case report demonstrates that PVS is an under-recognized complication of cardiovascular surgery and should be considered in all patients presenting with respiratory symptoms after a cardiac procedure.
Pulmonary vein stenosis (PVS) is an uncommon occurrence in adults, but one that carries significant morbidity and mortality. This entity can be secondary to neoplastic or non-neoplastic infiltration, extrinsic compression or iatrogenic intervention.1,2
PVS is characterized by progressive lumen size reduction of one or more pulmonary veins which, when significant, can raise lobar capillary pressure, leading to signs and symptoms such as shortness of breath, cough, and hemoptysis.3 Therefore, PVS can be initially diagnosed as pneumonia, malignancy or other parenchymal lung diseases, leading to delay of care and unnecessary invasive diagnostic tests.4,5 It is essential to consider the possibility of the disease in patients at-risk to guarantee early detection and treatment.
This article aims to describe a case of PVS that was primarily misdiagnosed as interstitial lung disease, and to review the literature that addresses the etiology, assessment, and management of this entity.
MethodsThe literature review was performed using four electronic databases (Pubmed, Cochrane, Scopus, and ISI-WOS) from inception until November 2019, involving the terms: “pulmonary vein stenosis”, “pulmonary venous infarction”, “interstitial lung disease”, “respiratory symptoms”. Further references from the case reports were considered. Exclusion criteria included commentaries and non-English language articles. Titles and abstracts were first examined to determine their relevance to the review. Duplicate articles between databases were initially identified and appropriately excluded.
ResultsThe identification results yielded 67 articles. After a careful analysis of the title and abstract, we included 23 articles. This information was summarized in a narrative review. We identified 4 case reports about PVS first diagnosed as primary lung disease (Table 1).
Articles reported in the literature about adult patients with PVS first diagnosed as primary lung disease (infection, malignancy and interstitial lung disease).
| Study, year, nr. Patients | Past medical history | Symptoms | High-resolution chest CT | Diagnostic approach | Treatment |
|---|---|---|---|---|---|
| Karthika R. Linga62015Case Report1 patient | Catheter ablation for AF 1 month before the onset of symptoms | Progressive dyspnea on exertion; dry cough | Bilateral ground-glass opacities; diffuse septal thickening; patchy consolidations in the left lung | (1) Video-assisted thoracoscopic surgery: severe congestion, including thickening of the interlobular and alveolar septa and accumulation of hemosiderin-laden macrophages in the alveoli(2) Transthoracic echocardiogram: normal mPAP (23mmHg) with a severely elevated right upper pulmonary vein velocity of >103cm/s(3) CT angiography of the heart: severe stenosis of all four pulmonary veins | Initial treatment with no improvement:(1) Antibiotics(2) Diuretics(3) High-dose steroidsSuccessful treatment:Balloon angioplasty with pulmonary vein stenting |
| Erin Fender52017Case Report1 patient | Catheter ablation for AF 7 months before the onset of symptoms | Dry cough; fatigue; hemoptysis | Right upper lobe consolidations with interlobular septal thickening | (1) Bronchoscopy and CT-guided needle biopsy: no evidence of infection or malignancy(2) CT pulmonary angiography: critical stenosis of the right superior pulmonary vein with associated intraparenchymal lung hemorrhage and infarction(3) Ventilation/perfusion scan: severe perfusion defect in the right upper and middle lobes | Itraconazole for presumed fungal pneumoniaSuccessful stenting of the right superior pulmonary vein |
| Fernández-Navarro72015Case Report1 patient | Catheter ablation for AF 4 months before the onset of symptoms | Dyspnea; sudden onset of intense left pleuritic pain | Peripheral alveolar consolidation in the left upper lobe and lingula, associated with pleural effusion | (1) Chest CT angiography: total occlusion of the left superior pulmonary vein(2) Cardiac catheterization: occlusion of the left superior pulmonary vein and critical stenosis of the left inferior pulmonary vein at the level of the ostium | Balloon angioplasty, followed by stent implantation |
| Tatsuyuki Kawahara82019Case Report1 patient | Catheter ablation for AF 5 months before the onset of symptoms | Chest pain; low-grade fever; hemoptysis | Migratory consolidations in the left upper lobe | (1) Transbronchial lung biopsy: fibrous thickening of the interlobular septa and oedematous thickening of the alveolar wall, congestive capillary proliferation (capillary haemangiomatosis)(2) Three-dimensional CT angiography and lung perfusion scintigraphy: total perfusion deficit of the left lung | Initial treatment with no improvement:(1) Antibiotics(2) High-dose steroidsSuccessful pericardial patch, venoplasty of the left pulmonary veins |
AF, atrial fibrillation; CT, computed tomography; mPAP, mean pulmonary arterial pressure.
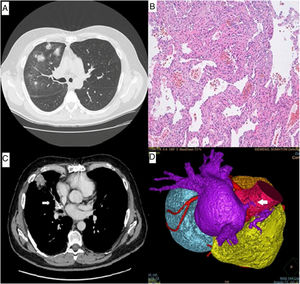
A 56-year-old man presented with progressive dyspnea on exertion and nonproductive cough for 3 months. He was a former smoker and had successfully undergone mitral valve reconstruction surgery due to ruptured chordae tendineae 4 months earlier. The computed tomography (CT) of the chest performed before surgery was normal, whereas six months later it revealed ground-glass opacities and patchy consolidations in the right upper and middle lobes (Fig. 1A).
(A) CT of the chest revealed ground-glass opacities and patchy consolidations in the right upper and middle lobes. (B) Histologically, the transbronchial lung biopsy material revealed thickening of the alveolar septa with rare lymphocytes and accumulation of hemosiderin macrophages in the alveoli. (C) Chest angiography showed stenosis of the right superior pulmonary vein (arrow). (D) A 3-dimensional computed tomographic reconstruction demonstrated severe stenosis of the right superior pulmonary vein with subtotal occlusion (arrow).
Bronchoalveolar lavage fluid showed a normal cell count without pathogens or neoplastic cells. After antibiotic and diuretic treatment, he underwent another CT scan that revealed migratory pulmonary infiltrates, but still confined to the right upper and middle lobes. Respiratory function tests, including diffusing capacity for carbon monoxide, were normal. A transbronchial lung biopsy was then performed and the pathological analysis of the specimens identified thickening of the alveolar septa with no evidence of vasculitis or organizing pneumonia, suggesting nonspecific interstitial pneumonia (NSIP) (Fig. 1B). Despite lacking any systemic symptoms of connective tissue disease, he was started on steroids based on a tissue diagnosis of NSIP. After 2 months of steroid treatment, no symptomatic improvement was reported. The CT scan was then repeated and continued to reveal ground-glass opacities in the right upper and middle lobes. Because of the temporal relation between the symptoms and cardiac surgery, lack of improvement despite steroid therapy and negative auto-immune laboratory testing, we further expanded our search for an alternative diagnosis.
A chest angiography was performed and revealed stenosis of the right superior pulmonary vein, which would later be confirmed with a CT angiogram of the heart (Fig. 1C and D). The previous transbronchial lung biopsy was carefully reviewed and showed signs of severe congestion including thickening of the alveolar septa with rare lymphocytes and accumulation of hemosiderin macrophages in the alveoli. Transthoracic echocardiography excluded pulmonary hypertension or other major abnormalities. The case was then discussed in a multidisciplinary meeting and the diagnosis of PVS was established. Clinical and imaging monitoring was maintained every 3–6 months. In the last follow-up visit, the patient reported no symptoms and showed radiological improvement.
DiscussionEtiologyCongenitalCongenital PVS is an exceptional abnormality (0.4% of congenital heart diseases) consequence of a failed incorporation of the common right and/or left pulmonary vein into the left atrium during the embryologic development of the vessel that leads to partial or complete obliteration of the pulmonary veins on one or both sides.2,3
AcquiredCurrently, radiofrequency ablation for atrial fibrillation (AF) has become the main cause of PVS. Incidence derived from recent studies reaches a mean and median of 2% and 3.1%, respectively.3 However, there are other clinical conditions predisposing to the obstruction of the central pulmonary veins, like mediastinal masses, such as solid neoplasms or bulky lymphoma, fibrosing mediastinitis and mediastinal granulomatous diseases.9,10 In addition, lung transplantation11,12 and lobectomy13 may result in PVS.
SymptomsWhen acquired after radiofrequency ablation or heart surgery, clinical manifestations usually appear 3–6 months after the procedure and are related to the number of pulmonary veins affected.3 Signs and symptoms include progressive exertional dyspnea, cough, chest pain fatigue, flu-like malaise, and hemoptysis.
In the largest published series, 33% of patients with PVS were initially diagnosed with bronchitis, pneumonia, or malignancy, leading to delayed care and unnecessary invasive diagnostic testing.4,5
DiagnosisLung parenchymal abnormalities are indirect evidence of PVS and can manifest as multifocal opacities, nodular lesions, unilateral effusions, and interstitial septal thickening.6 Image techniques are essential to reach a final diagnosis and decide on an appropriate therapy.
EchocardiographyTransesophageal echocardiography (TEE) is a useful tool for PVS investigation. The transthoracic echo window is seldom satisfactory for the evaluation of the pulmonary venous flow in adults. PVS is suspected if peak flow velocity exceeds 1.0ms−1 and/or if pulmonary vein diameter is <5mm.9
Contrast-enhanced chest CTChest CT allows for assessment of the extension of mediastinal neoplastic and non-tumoral diseases infiltrating or compressing the pulmonary veins and enables the diagnosis of PVS after radiofrequency ablation by directly depicting vessel diameter (significant stenosis >50%).3
The main benefits of CT are short examination time, multiplanar views, high spatial resolution, and providing a three-dimensional (3D) data set, whereas disadvantages include patient exposition to ionizing radiation and need of intravenous iodine contrast agents that might impair renal function in vulnerable individuals.3,9
Magnetic resonance imaging (MRI)MRI can be used to image the pulmonary veins.14,15 Many different techniques have been used, including traditional contrast-enhanced MRI and, more recently, time-resolved magnetic resonance venography.1 MRI, like CT, can provide a 3D data set but has the advantage of not using ionizing radiation. However, its spatial resolution is slightly inferior to that of CT, it requires a longer scanning time, and it may be contraindicated in patients with metal implants.
Ventilation/perfusion scanThe ventilation/perfusion scan is usually performed for the detection of pulmonary embolism but is also reported to serve as an effective screening tool for the detection of hemodynamically relevant PVS.16,17 This exam, however, is not valuable for the etiological diagnosis of PVS and may be altered in other pathologies that decreased lobar perfusion (i.e., pulmonary thromboembolism). It is not suitable for detection of <50% stenosis and may be inaccurate if significant compensating ipsilateral pulmonary vein flow is present.3,1
Management and follow upAny patient with a history of radiofrequency ablation for AF or other cardiac surgery presenting with new-onset cough, chest pain, fatigue, or hemoptysis should be considered to have PVS until proven otherwise. The radiologist also needs to be made aware of the ablation history so the study is planned appropriately for pulmonary vein assessment.5
Mild and asymptomatic PVS may not need intervention. However, clinical and image surveillance every 3–6 months is advised, as the disease can evolve over time. Some authors recommend clinical and imaging monitoring in patients with 50%-85% stenosis, while others promote angioplasty if a single stenosis or a cumulative stenosis index (average stenosis of the pulmonary veins of one site) >75%.3,18–21
Surgery or transcatheter therapy are the preferred approaches in most congenital or acquired significant symptomatic PVS. However, evidence of treatment of PVS due to extrinsic compression, infiltration or cardiac surgery is restricted to case reports and the therapeutic decision is usually made subject to individual aspect.22
Early recognition of PVS is essential because stenosis can progress rapidly to complete occlusion. Late diagnosis can result in worsening of the underlying inflammation and progressive loss of the lumen.20 Lung infarction is another possible consequence. As the vein narrows, it becomes more difficult to deploy a large stent and the risk for restenosis increases. In completely occluded veins, the rate of successful angioplasty or stenting is substantially reduced.5
ConclusionsPVS should be considered in all patients presenting with respiratory symptoms after cardiac surgery or radiofrequency ablation. Imaging techniques, such as CT angiography, play a fundamental role in the diagnosis and management of PVS, thanks to their good anatomical resolution, rapid results, and widespread availability.
Conflicts of interestThe authors have no conflicts of interest to declare.