Pulmonary myxoma is an extremely rare benign neoplasm. It is mostly parenchymal but may occasionally occur within the tracheobronchial tree. There are very few reports of endobronchial myxoma.
Case reportWe describe a case of endobronchial myxoma in a 40-year-old female patient with a history of asthma and repeated right-sided pneumonia. Thoracic computed tomography (CT) showed medium lobe atelectasis. Fiber optic bronchoscopy revealed a polypoid, well-circumscribed tumor, causing total obstruction of the medium lobe bronchus. Biopsy of the mass was non-diagnostic. Further study included a positron emission tomography (PET) which demonstrated low metabolic activity of the tumor and no evidence of neoplasia in other location. The patient was submitted to a medium lobectomy and microscopic examination of the tumor revealed myxoid stroma with lobulated pattern, elongated and stellate cells, compatible with myxoma.
ConclusionPulmonary myxoma is extraordinary rare and endobronchial location is very few reported in medical literature.
Os mixomas pulmonares são tumores benignos muito raros e quase sempre de localização parenquimatosa, mas ocasionalmente podem ocorrer na árvore traqueo-brônquica. Estão descritos raros casos de mixomas pulmonares endobrônquicos na literatura médica.
Caso clínicoDoente do sexo feminino, de 40 anos, com história de asma e pneumonias de repetição à direita. A tomografia computadorizada (TC) do tórax revelou atelectasia do lobo médio. A broncofibroscopia revelou lesão tumoral polipóide, de limites bem definidos, causando obstrução total do brônquio lobar médio. A biopsia do tumor não foi diagnóstica. A tomografia por emissão de positrões (PET) demonstrou atividade metabólica baixa do tumor e ausência de evidência de malignidade noutros locais. A doente foi submetida a lobectomia média e o exame microscópico do tumor revelou um padrão lobular com células alongadas e estreladas, tipo fibroblasto, com estroma mixoide intercelular abundante, compatível com mixoma pulmonar.
ConclusãoO mixoma pulmonar é muito raro e a sua localização endobrônquica está descrita em poucos casos na literatura médica.
Myxomas are benign mesenchymal tumors and the most common primary tumor at all ages in the heart, especially in the left atrium. Fatigue, syncope or arrhythmias are the most commonly associated clinical features. In 18% of cases the tumor is located in the right atrium, often diagnosed after recurrent pulmonary embolism.1 Histologically, myxomas are characterized by proliferation of fibroblast-like, elongated and stellate cells, dispersed in a myxoid stroma, features often common in several types of sarcomas.2 The term myxoma was introduced by Virchow to describe a tumor that mimics the structure of the Wharton jelly of the umbilical cord, and it is now recognized that skin, soft tissue and bone tumors may acquire similar myxoid appearance.3 Its pulmonary localization is very rare, and it is usually parenchymal, but may occur within the tracheobronchial tree. Few cases with endobronchial location2–4 are described in medical literature. The association of myxoma with other synchronous tumors, including primary lung adenocarcinoma, is also reported.5 The authors present a case of primary lung endobronchial myxoma.
Case reportThe patient was a 42 years-old female, ex-smoker since June 2007 (5 pack-years), working in the textile industry since the age of thirteen. She had a history of asthma from childhood usually treated with inhaled bronchodilators, under follow-up in the Department of Pulmonology. In June 2006 she had uncomplicated middle-lobe pneumonia, treated as an outpatient. In the past 3 years, recurrent respiratory infections became frequent. Significant family history of asthma (grandparents, father and brother) was also reported.
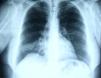
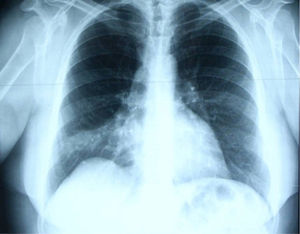
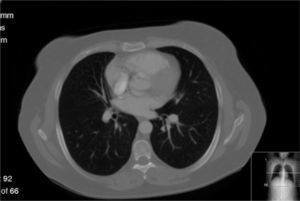
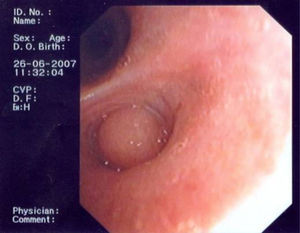
In June 2007 started complaining of cough with mucous sputum and wheezing. On physical examination, wheezing on pulmonary auscultation in the lower half of the right hemithorax was noted, with no signs of respiratory distress. Chest radiography showed a triangular-shaped opacity in the lower right lung region (Fig. 1). CT scan revealed atelectasis of the middle lobe and a nodular lesion in the middle lobar bronchus with 6mm diameter (Fig. 2). No abnormalities were detected through biochemical and haematological analysis. Lung function evaluation revealed mild obstructive syndrome. Fiber-optic bronchoscopy disclosed a polypoid, well-circumscribed, highly vascularized lesion, causing total obstruction of the medium lobe bronchus (Fig. 3). Its morphological appearance suggested a carcinoid tumor. Biopsy was not performed due to its associated bleeding risk and the patient underwent rigid bronchoscopy. Pathological examination of the tumor biopsy was inconclusive.
The patient was referred to thoracic surgery for evaluation, which included a positron emission tomography (PET) that revealed no focus of abnormal metabolic activity. The patient was submitted to middle-lobe lobectomy, without complications.
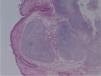
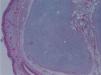
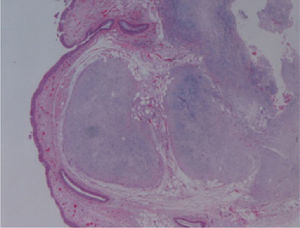
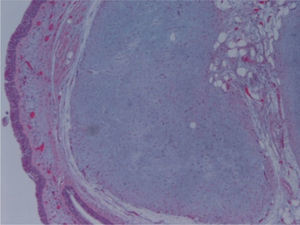
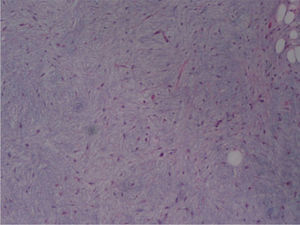
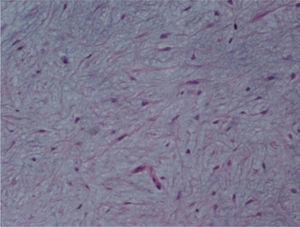
The macroscopic pathological exam revealed the middle lobe with 37.2g and 9×7×2.5cm in size, with a 0.5cm bronchial segment and a 5mm nodule with myxoid appearance and greyish color. Microscopically it was a benign subepithelial tumor with myxoid lobulated pattern, fusiform and stellate stromal cells, without atypia. Stroma was positive for Alcian Blue and stromal cells were PAS- and Vimentin-positive. The presence of cartilage in serial sections was not documented, even with immunohistochemistry. These morphologic features were compatible with primary pulmonary myxoma (Figs. 4–7).
Detail of Fig. 4.
Detail of Fig. 6, showing stellate with no atypia, mixoid stroma, without cartilaginous differentiation.
CT scan reassessment confirmed the absence of the middle lobe by surgical excision, without other significant changes. The patient is currently asymptomatic, after 3 years of follow-up by the Department of Pulmonology.
DiscussionMyxomas are benign mesenchymal tumors that may occur in many locations. The most common is the primary cardiac myxoma, which occurs mostly in adults, accounting for 30–50% of all cardiac tumors. Although most are sporadic, familial cardiac myxomas have an autosomal dominant transmission and constitute 7% of cardiac myxoma cases. The Carney complex refers to the association between atrial and extra-cardiac myxomas (with involvement of multiple sites) or Cushing syndrome.6,7 About 71% of myxomas occur in the heart, 41% in the skin and 7% in the oral cavity (usually in the palate).8 Myxomas in other locations than the heart are extremely rare, with few cases described in medical literature. The clinical presentation, treatment and outcome are well documented in relation to cardiac myxoma.1
The incidence of synchronous benign and malignant lung tumors is described, but the actual association between these entities is not known. Myxomas usually do not metastasize, however, metastasis to the brain, bone and soft tissues and local recurrence are described9–12 and attributed to emboli or sarcomatous origin.13 Therefore a long follow-up is usually recommended.14
In our case the myxoma was detected after recurrent respiratory infections and worsening of dyspnoea in a patient with a history of asthma. The presence of an endobronchial tumor was the root of poor control of the asthma and recurrent respiratory infections. Given the suspicion of malignancy, surgical excision of the lesion was performed. Microscopic examination of the tumor disclosed stellate cells in a myxoid stroma, containing thin-walled vessels, typical of myxoma. Microcystic architecture, nuclear pleomorphism and mitotic activity were absent. No epithelial, cartilage or other mesenchymal differentiation showed up in immunohistochemical studies. Cytologic and immunohistochemical features, such as the presence of myxoid stroma and absence of chondromatous or bronchial-like epithelial differentiation, made it possible to confidently make the differential diagnosis with the most frequent benign mesenchymal tumor in the lung, the hamartoma.
The benign clinical course is similar to other cases of primary pulmonary myxoma,2–4,15,16 without recurrence of the disease after a long follow-up. Clinical presentation is related to tumor location. Endobronchial tumor might cause wheezing, cough and recurrent infection, whereas peripheral nodule may be incidentally found.16 The presence of myxoma stroma, thin-walled vessels and stellate cells, and the absence of epithelial, chondroid, lipomatous or neural differentiation, are the hallmarks of histological diagnosis of lung myxoma. These features are common to other cases reported.2–4,15,16
In medical literature, data regarding treatment, evolution and prognosis of lung primary myxomas is very limited, and for this reason we present our case.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rolo R, et al. Mixoma endobrônquico – Caso clínico. Rev Port Pneumol. 2012. doi:10.1016/j.rppneu.2011.12.004