OSAS (obstructive sleep apnea syndrome) is defined by recurrent episodes of upper airway obstruction during sleep, causing multiple clinical consequences. Literature review suggests that OSAS induces a spectrum of abnormalities in neural, hormonal and vascular regulation that contribute to the development of ED (erectile dysfunction).
The aims of this study were to estimate the prevalence of ED in OSAS patients and evaluate its determinants.
Methods62 patients from Hospital S. João Sleep Laboratory with newly diagnosed OSAS were included in the study and answered the IIEF-5 (international index erectile function 5 item version) questionnaire.
ResultsThe prevalence of ED in OSAS patients was 64.4%. Age and diabetes constituted themselves as independent risk factors for more severe degrees of ED: OR=1.226 (95% CI: 1.062–1.415) and OR=31.205 (95% CI: 1.222–796.557), respectively. Compared with nonsmokers, ex-smokers group revealed a positive association with ED: OR=4.32 (95% CI: 1.09–17.11). Hypertension and ACEI (angiotensin converting enzyme inhibitors) or ARB (angiotensin II receptor blockers) therapy were also correlated to ED symptoms: OR=3.25 (95% CI: 1.09–9.65) and 7.39 (95% CI: 1.52–35.99), respectively.
No association was found relating BMI (p=0.254), alcoholic habits (p=0.357), acute myocardial infarction (p=0.315), dyslipidemia (p=0.239), metabolic syndrome (p=0.215) and ED.
OSAS severity was not associated with ED in our sample.
ConclusionsThe prevalence of ED in OSAS patients is high. ED determinants in our sample were age and diabetes. Past smoking habits, hypertension and ACEI/ARB therapy also revealed a statistically significant association with ED.
A SAOS (síndroma de apneia obstrutiva do sono) define-se pela ocorrência frequente de obstrução da via aérea superior durante o sono, com múltiplas consequências clínicas. Estudos anteriores sugerem que a SAOS provoca alterações na regulação neural, hormonal e vascular que contribuem para o desenvolvimento de DE (disfunção erétil).
Este estudo tem como principais objetivos estimar a prevalência da DE numa amostra de doentes com SAOS e avaliar os seus determinantes.
MétodosForam incluídos 62 doentes do Laboratório do Sono do Hospital S. João com diagnóstico recente de SAOS, que responderam ao questionário IIEF-5 (International Index Erectile Function-5 Item version).
ResultadosA prevalência da DE em pacientes com SAOS foi de 64,4%. A idade e a diabetes constituíram fatores de risco independentes para graus avançados de DE: OR=1,226 (IC 95%:1,062–1,415) e OR=31,205 (IC 95%:1,222–796,557), respetivamente. Comparados com pacientes fumadores, o grupo de pacientes ex-fumadores revelou associar-se à DE: OR=4,32 (IC 95%:1,09–17,11). A hipertensão e o tratamento com IECAS (inibidores da enzima conversora da angiotensina) ou ARA (antagonistas dos recetores da angiotensina) evidenciaram uma associação com DE: OR=3,25 (IC 95%:1,09–9,65) e 7,39 (IC 95%:1,52–35,99), respetivamente.
Não foi encontrada nenhuma relação no que diz respeito ao IMC (p=0,254), hábitos alcoólicos (p=0,357), enfarte agudo do miocárdio (p=0,315), dislipidemia (p=0,239), síndrome metabólico (p=0,215) e DE.
A gravidade da SAOS não se encontra associada a DE na amostra estudada.
ConclusõesA prevalência da DE em doentes com SAOS é elevada. Os determinantes da DE na amostra estudada foram a idade e a diabetes. Ex-fumadores, hipertensão e tratamento com ACEI/ARB também revelaram uma associação estatisticamente significativa com a DE.
Erectile dysfunction (ED) is defined as the consistent inability to obtain and/or maintain a penile erection which is sufficient to permit satisfactory sexual intercourse.1,2 The prevalence of ED is estimated at 48% among Portuguese men aged 40–69 years old.3
Obstructive sleep apnea syndrome (OSAS) is characterized by repetitive collapse of the upper airway due to the laxity of pharyngeal dilator muscles4 during sleep.
OSAS affects 4% of men between 30 and 60 years,5 but it is believed that the proportion of clinically diagnosed OSAS is underestimated.6–8 This is one of the most important medical conditions to have been identified in the last 50 years.9 It is related to increased morbidity and mortality due to clinical complications such as hypertension,10 congestive heart failure,11 acute myocardial infarction,12 stroke,13 diabetes,14 cognitive dysfunction15 and depression.16
In every REM sleep stage most men experience a sleep related erection (SRE),17 an event that ensures functional and morphological integrity to erectile tissue.18 In OSAS, intermittent hypoxic events and sleep fragmentation limit SRE, with serious consequences for erectile physiology.19–22 The literature review also defines hormonal,23–26 neural,27–30 endothelial31–35 and psychogenic16 mechanisms to explain ED complaints in OSAS.
OSAS and ED may also be connected through co-morbidities such as hypertension and diabetes.21
As there are some inconsistencies in regard to the prevalence data,18 the main purpose of this study was to estimate the prevalence of ED in a population of OSAS patients sent to Hospital S. João for diagnosis and follow-up. Additionally, clinical and demographic information was collected to obtain the ED determinants in our population.
MethodsStudy populationBetween 28 September and 31 December 2010, all men admitted (n=207) to S. João Hospital Sleep Laboratory for a first medical appointment because of suspected OSAS, were invited to participate in the present study. Each patient received written information about the study, an Informed Consent form and an IIEF-5 questionnaire for them to complete.
Ninety-five patients returned the completed questionnaire, 33 patients were excluded after sleep study as they did not present OSAS and one was excluded because of a previous diagnosis of ED.
Information about BMI, previous medical history (diabetes, hypertension, stroke, acute myocardial infarction) and usual pharmacological therapy was obtained from medical files.
This study was approved by S. João Hospital Ethics Committee.
IIEF-5 (International Index Erectile dysfunction – 5 item version)IIEF-5 is a simple and useful questionnaire for screening patients with ED.36 Designed as a simplified version of IIEF, it consists of 5 questions regarding erectile function and satisfaction37 (Appendix A). The possible scores for IIEF-5 range from 5 to 25, and ED was classified into five categories based on the scores obtained: absent (22–25), mild (17–21), mild to moderate (12–16), moderate (8–11), and severe (5–7).
It was validated to be used in Portuguese.38
Polygraphic cardiorespiratory sleep study (PCSS)OSAS is defined by the presence of at least 5 obstructive respiratory events (apneas, hypopneas or respiratory effort related arousals) per hour of sleep in association with daytime sleepiness, loud snoring, witnessed breathing interruptions or waking up due to gasping or choking.39 The presence of 15 or more obstructive respiratory events in the absence of sleep related symptoms is also sufficient for the diagnosis.39
Polysomnography is routinely indicated for the diagnosis of sleep related breathing disorders,39–42,44,43 but its use is limited by high costs and waiting lists.45
An overnight PCSS is an alternative to Polysomnography when there is high pre-test probability.39 PCSS is made up of a portable monitor device46 that records oronasal airflow (measured by nasal cannula), arterial oxygen saturation (measured by finger pulse oximetry), pulse rate, upper limb, abdominal and thoracic movements, body position and snoring.47
All participants underwent PCSS with ApneaLink®, AlphaScreen Pro®, EMBLETTA® or Stardust® devices, which calculate the number of apneas (episodes of ≤20% of previous airflow with at least 10s of duration) and hypopneas (episodes showing 20–50% of the previous airflow, with at least 10s of duration joined with a 4% dip in oxygen saturation) per hour of estimated sleep time. It also provides information on desaturations >4% per hour of estimated sleep time (oxygen desaturation index).
Our analysis focused on apnea–hypopnea index (AHI), oxygen desaturation index (ODI), minimum and medium oxygen saturation.
According to established criteria,39 the severity OSAS was stratified according to AHI value: mild (5–15), moderate (16–29) and severe (≥30).
Statistical analysisData analysis was performed with SPSS® (Statistical Package for Social Sciences), 18.0 version.
A frequency analysis was used to describe the population. The Chi-square test was used to determine the association between categorical variables and Kruskall–Wallis test to define any association between continuous and categorical variables. Multivariate logistic regression was used to evaluate the effect of each variable adjusted to other possible confounding factors.
Every association with p<0.05 was considered statistically significant.
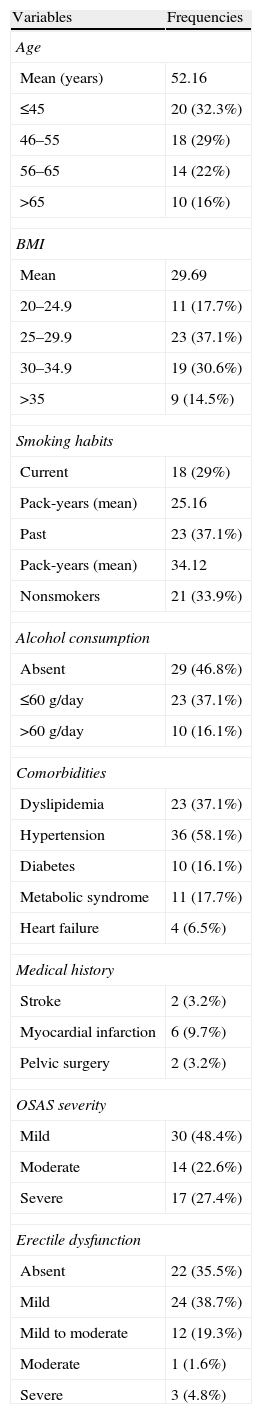
ResultsThe general characteristics of the population are described in Table 1. The mean age was 52 years. Of 62 patients studied, 28 (45.1%) were obese, 23 (37.1%) had dyslipidemia, 11 (17.7%) metabolic syndrome (MS), 36 (58.1%) arterial hypertension, 10 (16.1%) diabetes and 4 (6.5%) heart failure. Previous acute myocardial infarction, stroke and pelvic surgery were identified in 2 (3.2%), 6 (9.7%) and 2 (3.2%) participants, respectively. Mild OSAS was diagnosed in 30 (48.4%), moderate OSAS in 14 (22.6%) and severe OSAS in 17 (27.4%) individuals. The ED prevalence was 64.4%, it was mild in 24 (38.7%), mild to moderate in 12 (19.3%), moderate in 1 (1.6%) and severe in 3 (4.8%) participants.
Population general description (n=62).
| Variables | Frequencies |
| Age | |
| Mean (years) | 52.16 |
| ≤45 | 20 (32.3%) |
| 46–55 | 18 (29%) |
| 56–65 | 14 (22%) |
| >65 | 10 (16%) |
| BMI | |
| Mean | 29.69 |
| 20–24.9 | 11 (17.7%) |
| 25–29.9 | 23 (37.1%) |
| 30–34.9 | 19 (30.6%) |
| >35 | 9 (14.5%) |
| Smoking habits | |
| Current | 18 (29%) |
| Pack-years (mean) | 25.16 |
| Past | 23 (37.1%) |
| Pack-years (mean) | 34.12 |
| Nonsmokers | 21 (33.9%) |
| Alcohol consumption | |
| Absent | 29 (46.8%) |
| ≤60g/day | 23 (37.1%) |
| >60g/day | 10 (16.1%) |
| Comorbidities | |
| Dyslipidemia | 23 (37.1%) |
| Hypertension | 36 (58.1%) |
| Diabetes | 10 (16.1%) |
| Metabolic syndrome | 11 (17.7%) |
| Heart failure | 4 (6.5%) |
| Medical history | |
| Stroke | 2 (3.2%) |
| Myocardial infarction | 6 (9.7%) |
| Pelvic surgery | 2 (3.2%) |
| OSAS severity | |
| Mild | 30 (48.4%) |
| Moderate | 14 (22.6%) |
| Severe | 17 (27.4%) |
| Erectile dysfunction | |
| Absent | 22 (35.5%) |
| Mild | 24 (38.7%) |
| Mild to moderate | 12 (19.3%) |
| Moderate | 1 (1.6%) |
| Severe | 3 (4.8%) |
BMI (body mass index); OSAS (obstructive sleep apnea syndrome); ED (erectile dysfunction) classification: absent (IIEF-5 22–25); mild (IIEF-5 17–21); mild to moderate (IIEF-5 12–16); moderate (IIEF-5 8–11); severe (IIEF-5 5–7).
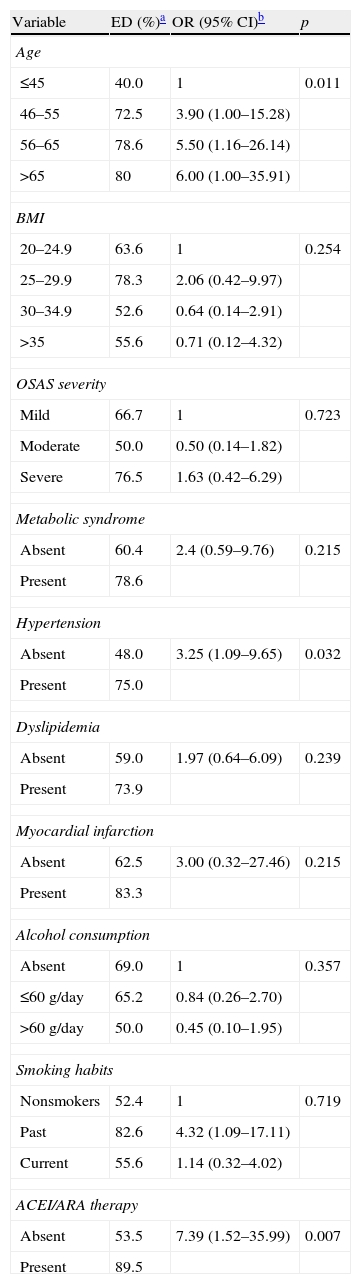
Univariate analysis results are shown in Table 2. Aging was found to be significantly related to ED: OR (95% CI) of 3.90 (1.00–15.28); 5.50 (1.16–26.14); and 6.00 (1.00–35.91) in categories 46–55, 56–65 and >65 years, respectively. Past smoking habits were associated with ED (OR 4.32 (1.09–17.11). Hypertension and ACEI (Angiotensin Converting Enzyme Inhibitors) or ARB (Angiotensin II Receptor Blockers) therapy revealed a statistically significant association with ED (OR 3.25 (1.09–9.65) and 7.39 (1.52–35.99)).
ED prevalence and association with clinical and demographic variables.
| Variable | ED (%)a | OR (95% CI)b | p |
| Age | |||
| ≤45 | 40.0 | 1 | 0.011 |
| 46–55 | 72.5 | 3.90 (1.00–15.28) | |
| 56–65 | 78.6 | 5.50 (1.16–26.14) | |
| >65 | 80 | 6.00 (1.00–35.91) | |
| BMI | |||
| 20–24.9 | 63.6 | 1 | 0.254 |
| 25–29.9 | 78.3 | 2.06 (0.42–9.97) | |
| 30–34.9 | 52.6 | 0.64 (0.14–2.91) | |
| >35 | 55.6 | 0.71 (0.12–4.32) | |
| OSAS severity | |||
| Mild | 66.7 | 1 | 0.723 |
| Moderate | 50.0 | 0.50 (0.14–1.82) | |
| Severe | 76.5 | 1.63 (0.42–6.29) | |
| Metabolic syndrome | |||
| Absent | 60.4 | 2.4 (0.59–9.76) | 0.215 |
| Present | 78.6 | ||
| Hypertension | |||
| Absent | 48.0 | 3.25 (1.09–9.65) | 0.032 |
| Present | 75.0 | ||
| Dyslipidemia | |||
| Absent | 59.0 | 1.97 (0.64–6.09) | 0.239 |
| Present | 73.9 | ||
| Myocardial infarction | |||
| Absent | 62.5 | 3.00 (0.32–27.46) | 0.215 |
| Present | 83.3 | ||
| Alcohol consumption | |||
| Absent | 69.0 | 1 | 0.357 |
| ≤60g/day | 65.2 | 0.84 (0.26–2.70) | |
| >60g/day | 50.0 | 0.45 (0.10–1.95) | |
| Smoking habits | |||
| Nonsmokers | 52.4 | 1 | 0.719 |
| Past | 82.6 | 4.32 (1.09–17.11) | |
| Current | 55.6 | 1.14 (0.32–4.02) | |
| ACEI/ARA therapy | |||
| Absent | 53.5 | 7.39 (1.52–35.99) | 0.007 |
| Present | 89.5 | ||
No association was found between dyslipidemia (p=0.239), metabolic syndrome (p=0.215), chronic therapy with beta blockers (p=0.217), calcium antagonists (p=0.827) and serotonin-selective reuptake inhibitors (p=0.250) and ED.
All patients with diabetes, stroke, cardiac failure and previous pelvic surgery referred to ED on questionnaire, so it was not possible to calculate odds ratio (OR) for these variables.
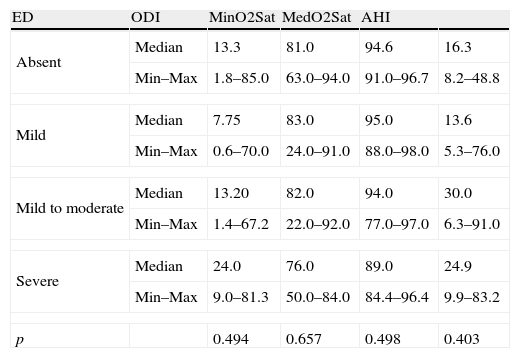
PCSS parameter analysis is described in Table 3. No association was verified between ED and OSAS severity measured by ODI, minimum and medium oxygen saturation and AHI (p=0.494, p=0.657, p=0.498 and p=0.403, respectively).
PCSS study parameter analysis according to ED severity.
| ED | ODI | MinO2Sat | MedO2Sat | AHI | |
| Absent | Median | 13.3 | 81.0 | 94.6 | 16.3 |
| Min–Max | 1.8–85.0 | 63.0–94.0 | 91.0–96.7 | 8.2–48.8 | |
| Mild | Median | 7.75 | 83.0 | 95.0 | 13.6 |
| Min–Max | 0.6–70.0 | 24.0–91.0 | 88.0–98.0 | 5.3–76.0 | |
| Mild to moderate | Median | 13.20 | 82.0 | 94.0 | 30.0 |
| Min–Max | 1.4–67.2 | 22.0–92.0 | 77.0–97.0 | 6.3–91.0 | |
| Severe | Median | 24.0 | 76.0 | 89.0 | 24.9 |
| Min–Max | 9.0–81.3 | 50.0–84.0 | 84.4–96.4 | 9.9–83.2 | |
| p | 0.494 | 0.657 | 0.498 | 0.403 | |
It was not possible to analyze the category “moderate DE” because it was composed only by one subject.
ED (erectile dysfunction) classification: absent (IIEF-5 22–25); mild (IIEF-5 17–21); mild to moderate (IIEF-5 12–16); moderate (IIEF-5 8–11); severe (IIEF-5 5–7).
ODI (oxygen desaturation index); MinO2Sat (minimum O2 saturation); MedO2Sat (medium O2 saturation); AHI (apnea–hypopnea index); Min–Max (minimum–maximum).
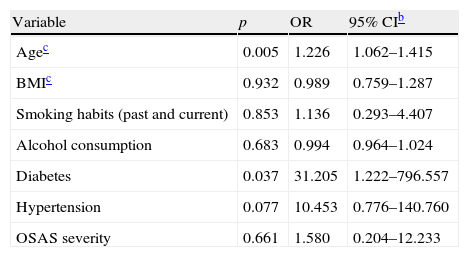
Multivariate analysis results are shown in Table 4. Age and diabetes were independent risk factors for more severe degrees of ED: OR (95% CI) of 1.226 (1.062–1.415) and 31.205 (1.222–796.557), respectively. There was no evidence that BMI (p=932), smoking habits (p=853), alcohol consumption (p=0.683), hypertension (p=0.077) and OSAS severity (p=0.661) were independent risk factors for ED.
Multivariate logistic regressiona on the association between some clinical variables and ED.
| Variable | p | OR | 95% CIb |
| Agec | 0.005 | 1.226 | 1.062–1.415 |
| BMIc | 0.932 | 0.989 | 0.759–1.287 |
| Smoking habits (past and current) | 0.853 | 1.136 | 0.293–4.407 |
| Alcohol consumption | 0.683 | 0.994 | 0.964–1.024 |
| Diabetes | 0.037 | 31.205 | 1.222–796.557 |
| Hypertension | 0.077 | 10.453 | 0.776–140.760 |
| OSAS severity | 0.661 | 1.580 | 0.204–12.233 |
OSAS (obstructive sleep apnea syndrome).
In 1981, Schmidt and Wise were the first to describe a relationship between ED and sleep disorders.48 After that, several studies confirmed the increased prevalence of ED in OSAS patients51–54: Guilleminault et al. reported ejaculatory dysfunction and decreased libido in 48% men with OSAS49; Hirshkowitz et al. verified that 91.3% patients with ED symptoms also had OSAS50; Seftel et al. concluded that 40% OSAS patients had ED.51 However, the association was rejected by Schiavi et al.52 In the present study, a 64.4% ED prevalence was found, which is consistent with most published material on the subject.
In our sample, ED was not associated with severity of OSAS. However it can be seen that the group with severe OSAS did have a higher ED prevalence (Table 2) and patients who reported severe ED had the worst results in sleep study (Table 3), which indicates that studies with higher sample size are needed to support the association Margel et al. concluded that ED is associated exclusively with severe OSAS.53 Furthermore, trials evaluating OSAS treatment found improvements in ED,54–58 which shows that there may be an association between both disorders, which is understandable given that patients on CPAP treatment show decreased hypoxia, improved endothelial function, decreased blood pressure and sympathetic hyperactivity, all of which can improve erectile function.
In this study, age proved to be an independent risk factor for ED, as suggested by the literature.59 It was also confirmed that the risk of ED increases with aging. It is already established that age has more impact on erectile function than the severity of OSAS.60
In addition, the data confirmed that Diabetes is an independent risk factor for ED. It can be seen that diabetic patients have a 31 times higher risk for more severe ED, which is supported by the effect of hyperglycemia on penile neurovascular structure.61
Arterial hypertension was associated with ED in the univariate analysis. However, an association with more severe ED was not confirmed by the multivariate analysis. This difference is probably due to a confounding effect of diabetes, suggested by the fact that 9 out of 10 diabetic patients also suffered from hypertension (data not shown in tables).
An association between MS and ED was not found. Nevertheless, it is possible that the classification used underestimated the actual proportion of affected patients since it was based on patient report and did not include triglycerides or cholesterol plasmatic measurements.62 As a marker of systemic endothelial dysfunction, dyslipidemia may be more prevalent in ED patients.63 Fibrats and Statins, lipid-lowering drugs, can also induce ED as a side effect,64 which was not observed in our sample.
Similarly, this study did not prove an association between BMI and ED, with patients in BMI interval 25–29 reporting more symptoms than those included in higher BMI categories. This is probably related to the fact that the BMI interval 25–29 included older patients (43% patients between 56 and 65 years and 50% of patients older than 65 years), compared to the other categories (data not shown in tables). In our sample, aging is an important confounding factor when analyzing BMI data.
Concerning smoking habits, only ex-smokers showed a statistically significant association with ED, probably due to the fact that this group reported having had heavier smoking habits (34 pack-years), compared to current smokers (25 pack-years). In a recent cross-sectional study65 smoking habits of more than 23 years or 20 cigarettes per day were significantly associated with ED.
Regarding chronic medication, an association was found between ACEI/ARB and ED. In fact, anti-hypertensive therapy is associated with adverse sexual effects, most commonly involving diuretics and beta-blockers.66 ACEI/ARB were the most frequently used drugs and its association with ED may be due to hypertension itself.
This study has some strong points in its favor. We used a validated instrument to diagnose ED in clinical settings, which was designed by urologists, and so added a multidisciplinary approach to OSAS. Patients with co-morbidities were not excluded, which made it possible to measure the impact on erectile function. The effect of chronic medication was also evaluated.
However, this study also has limitations. Although it was a representative population for estimating prevalence, we analyzed multiple clinical variables, some of them shared by our small number of subjects, which may have prevented us from establishing more statistical significant associations. The size of our sample did not lend itself to such extended analysis. The study was small because there was a high refusal rate for patient participation (54%), possibly related to the embarrassment about describing such an intimate subject.
For future research larger scale studies are needed to be able to evaluate multiple clinical variables. Among the extended co-morbidities inherent to OSAS it is extremely important not to neglect complications such as ED. If we can use a bio-psychosocial approach with our patients, it will certainly have greater impact.
ConclusionsED is very common among patients with OSAS and may be under recognized because patients do not spontaneously report the problem. Several factors are associated with ED among patients with OSAS and those include age, diabetes, past smoking habits, hypertension and ACEI/ARB therapy.
Conflicts of interestThe authors have no conflicts of interest to declare.
I would like to thank Professor Marta Drummond, first for joining this project, and then for her tireless technical, bibliographical and motivational support.
I would also express my gratitude to Dr. Francisco Botelho for helping with the statistical analysis.
I would like to thank Sleep Laboratory professionals (clinicians, technicians and secretaries) for their cooperation in the collection of all relevant clinical information.
Finally, I would like to thank my parents, Joana Lascasas and Roberto Moreira for all their technical and emotional support.
- (1)
How would you rate your level of confidence in achieving and maintaining an erection?
- 1.
Too low/none
- 2.
Low
- 3.
Normal
- 4.
High
- 5.
Too high
- 1.
- (2)
When you achieve erection by sexual stimulation, how often is that erection sufficiently firm for penetration?
- 1.
Almost never/never
- 2.
A few times (less than half of the times)
- 3.
Sometimes (about half of the times)
- 4.
Often (more than half of the times)
- 5.
Almost every time/always
- 1.
- (3)
During sexual intercourse, how many times have you managed to maintain an erection after penetration?
- 1.
Almost never/never
- 2.
A few times (less than half of the times)
- 3.
Sometimes (about half of the times)
- 4.
Often (more than half of the times)
- 5.
Almost every time/always
- 1.
- (4)
During sexual intercourse, is it difficult to maintain an erection until the end of sexual activity?
- 1.
Extremely difficult
- 2.
Very difficult
- 3.
Difficult
- 4.
Slightly difficult
- 5.
Not difficult
- 1.
- (5)
When you try to have attempt sexual intercourse, how often are you successful?
- 1.
Almost never/never
- 2.
A few times (less than half of the times)
- 3.
Sometimes (about half of the times)
- 4.
Often (more than half of the times)
- 5.
Almost every time/always
- 1.
Please cite this article as: Santos T. Disfunção eréctil na síndrome de apneia obstrutiva do sono – Prevalência e determinantes. Rev Port Pneumol. 2011. doi:10.1016/j.rppneu.2011.10.004.