To evaluate the dose–response relationship between smoking load and cardiopulmonary fitness, as measured with cardiopulmonary exercise testing (CPET), in adult smokers free of respiratory diseases.
MethodsAfter a complete clinical evaluation and spirometry, 95 adult smokers (35 men and 60 women) underwent CPET on a treadmill.
ResultsThe physiological responses during CPET showed lower cardiorespiratory fitness levels, regardless of smoking load, with a peak V′O2 lower than 100% of the expected value and a lower maximum heart rate. We observed a significant moderate negative correlation between smoking load and peak V′O2. The smoking load also presented a significant negative correlation with maximum heart rate(r=−0.36; p<0.05), lactate threshold(r=−0.45; p<0.05), and peak ventilation(r=−0.43; p<0.05). However, a dose–response relationship between smoking load quartiles and cardiopulmonary fitness was not found comparing quartiles of smoking loads after adjustment for age, sex and cardiovascular risk.
ConclusionThere appears to be no dose–response relationship between SL and cardiopulmonary fitness in adult smokers with preserved pulmonary function, after adjusting the analysis for age and cardiovascular risk. Our results suggest that smoking cessation might be useful as the primary strategy to prevent cardiopulmonary fitness decline in smokers, regardless of smoking load. Thus, even a very low dose of tobacco use must be avoided in preventive strategies focusing on becoming people more physically active and fit.
Tobacco use continues to be the leading global cause of preventable deaths.1 Smoking affects health among young smokers without established chronic disease.2 Smoking increases the risk of developing respiratory and cardiovascular diseases, and it is responsible for causing many types of cancer, even in non-smokers exposed to second-hand tobacco smoke (SHS). When smoking, a person inhales an average of 2500 toxic substances leading to symptoms such as increased mucus production, airway inflammation, infections, and decreased muscular function.3
Smoking is associated not only with lower physical activity, but also with impaired cardiorespiratory fitness and heart rate variability.4 The best way to determine cardiorespiratory fitness is through cardiopulmonary exercise testing (CPET). One of the variables used to determine the functional cardiorespiratory capacity is the measurement of the pulmonary oxygen uptake (V′O2) at peak exercise intensity.
Smoking load (SL), expressed in pack-years, is widely used as a simple way to quantify current tobacco use. An SL greater than 15 pack-years should have detailed screening for respiratory diseases, such as chronic obstructive pulmonary disease (COPD).5 Lung cancer screening is recommended for individuals with an SL greater than 30 pack-years.6 However, the health effects of lower SL are not fully understood. Although there is detailed knowledge of the negative effects of smoking, there are few studies on the dose–response relationship of SL and cardiorespiratory fitness. The objective of this study was to evaluate the dose–response relationship between SL and cardiopulmonary fitness through CEPT in adult smokers with preserved pulmonary function.
MethodsIn this cross-sectional study, 95 adult smokers (35 men and 60 women) underwent CPET on a treadmill, after a complete clinical evaluation and spirometry. Participants were selected from the EPIMOV Study (Epidemiology and Human Movement Study). Briefly, the EPIMOV Study, is a cohort study with the main objective of investigating the longitudinal association between sedentary behavior and physical inactivity with the occurrence of hypokinetic diseases, especially cardiorespiratory diseases. The volunteers were recruited through dissemination in social networks, folders displayed in the universities of the region, local magazines and newspapers. Inclusion criteria for this study were male or female aged between 18 and 90 years and being free from self-reported physician-diagnosed cardiac or pulmonary disease. Exclusion criteria were orthopedic problems, recent respiratory infections, unstable or stable angina in the last four weeks, recent myocardial infarction, angioplasty or cardiac surgery in the last three months and spirometric abnormalities. We have excluded participants considering impaired functional vital capacity (FVC<80% predicted) and/or low relationship between forced expiratory volume in the 1st second and FCV (FEV1/FVC ratio≤0.7 in absolute value).7–9 In order to calculate the predicted spirometric variables, Brazilian reference values10 were used. The participants were informed about the possible risks and discomforts of the procedures proposed in the present study and signed a consent form. The Ethics Committee for Research in Humans of the local university approved this study by the 186.796 protocol.
Clinical evaluationThe height and weight of the subjects were measured and the body mass index (BMI) was calculated. Personal and demographic data were collected (e.g., sex, age, education, home address). In addition, participants answered the physical activity readiness questionnaire (PAR-Q) in order to evaluate some possible contraindication for CPET11; questions about respiratory disorders based on the American Thoracic Society (ATS) questionnaire12 to investigate pollutants exposition, history of asthma and smoking status; and cardiovascular disease (CVD) risk stratification was performed according to the American College of Sports Medicine (ACSM).13 We investigated the presence of self-reported major risk factors for CVD, including age (male≥45 years; female≥55 years); family history of premature coronary heart disease (CHD) (definite myocardial infarction before 55 years old in father or 65 years old in mother or other first-degree relative); systemic arterial hypertension; diabetes; dyslipidemia and current cigarette smoking.
SpirometryThe forced vital capacity (FVC) was determined using a calibrated spirometer (Quark PTF; COSMED, Pavonadi Albano, Italy), following the criteria of the American Thoracic Society (ATS).14 The forced expiratory volume in 1-s (FEV1) was measured, and then the FEV1/FVC ratio was calculated. All spirometric values were measured in absolute and percentage of normal values by using reference values for the Brazilian population.10
Cardiopulmonary exercise testingAll participants were informed about the preparatory procedures prior to CPET. Several recommendations were standardized, such as not smoking on the assessment day, not performing intense exercise on test day and avoiding coffee, tea or on test day. CPET was done on a treadmill (ATL, Inbrasport, Curitiba, Brasil) by using a ramp protocol. Pulmonary oxygen uptake (V′O2), carbon dioxide output (V′CO2) and minute ventilation (VE) were recorded using a computerized system for cardiopulmonary exercise testing, periodically calibrated following the manufacturer's recommendations (Quark PTF; COSMED, Pavona di Albano, Italy). Heart rate was monitored during CPET with a 12-lead EKG (C12x; COSMED, Pavona di Albano, Italy). The V′O2 equivalent to the lactate threshold was obtained through a gas exchange technique, visually inspecting the VCO2/V′O2 slope inflection point (v-slope) and by using the oxygen (VE/V′O2) and carbon dioxide (VE/V′CO2) ventilatory equivalents.15 The data were averaged every 15-s. The average of the last 15s at the end of the test, immediately before the recovery phase, was considered the peak V′O2. Subjects with less than 83% of the predicted peak V′O2 were considered exercise intolerant.16
Statistical analysisThe sample size was calculated assuming a minimum clinically significant difference of 442mL/min in peak VO2 during the CPET in healthy individuals (i.e., the lower limit of normal).17 This was selected because the standard deviation of peak V′O2 in healthy adults is about 400mL/min.17 From this, the probability of alpha and beta errors was set at 0.05 and 0.20, respectively, establishing a minimum sample size of 17 participants in each of the quartiles for CPET to detect a 442mL difference in peak V′O2. Thus, a sample size of 68 subjects was calculated in our study.
Data were analyzed and normal variables presented as the mean±standard deviation, or as median (interquartile range) for non-normal variables. The correlation between SL and the data obtained in the CPET were evaluated with the Pearson or Spearman correlation coefficient, according to the normality of the variables. The SL was retrospectively categorized into the 25th, 50th, and 75th percentiles. Peak V′O2 was compared using multivariate analysis of variance (MANOVA) and adjusted for the following main confounding variables: age, sex, systemic arterial hypertension, diabetes mellitus, dyslipidemia, obesity, and physical inactivity. A p value <0.05 was considered significant.
ResultsThis study evaluated 95 smokers without pulmonary disease. The subjects were, on average, middle-aged, with a BMI indicating obesity and normal spirometry (Table 1). Restrictive impairment was present in 13 (13.7%) subjects. Cardiovascular risk factors were present: 13% were diabetic, 21% had systemic arterial hypertension, 28% had dyslipidemia, 46% were obese, and 69% were physically inactive.
General characteristics of the study subjects and physiological responses observed in the cardiopulmonary exercise testing.
| Mean±SD | |
|---|---|
| Age (years) | 45±12 |
| Weight (kg) | 83±23 |
| Sex (Male/Female) | 35/60 |
| Height (m) | 1.63±0.98 |
| BMI (Kg/m2) | 30.7±7.5 |
| FVC (L) | 3.32±3.03 |
| FVC (% of predicted) | 90.5±13.8 |
| FEV1 (L) | 2.68±0.91 |
| FEV1 (% of predicted) | 88.7±15.3 |
| FEV1/FVC (%) | 80.0±7.4 |
| Peak VO2 (ml/min) | 2038±774 |
| Peak VO2 (ml/min/Kg) | 24±8 |
| Peak VO2 (% of expected) | 88±15 |
| Anaerobic threshold (ml/min) | 1376±505 |
| Anaerobic threshold (% of the predicted peak VO2) | 60±12 |
| Rate of gas exchange (VCO2/VO2) | 1.13±0.11 |
| Maximum heart rate (bpm) | 153±19 |
| Maximum heart rate (% of predicted) | 88±8 |
| Maximum minute ventilation(L/min) | 66±25 |
SD, standard deviation; BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; VO2, oxygen uptake; VCO2, carbon.
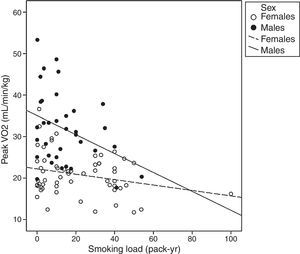
In general, the physiological responses during the CPET represented a lower cardiorespiratory fitness level, as indicated by peak V′O2 lower than 100% of the expected and a lower maximum heart rate (Table 1). Thirty-two subjects (33.6%) had exercise intolerance. A significant moderate negative correlation between SL and the peak V′O2 was detected (Fig. 1). The SL also showed significant correlation with maximum heart rate (r=−0.36; p<0.05), lactate threshold (r=−0.45; p<0.05) and peak VE (r=−0.43; p<0.05).
The SL quartiles were divided as ≤3, between 3 and 12, between 12.1 and 32 and >32 pack-years. The participants with an SL greater than 32 pack-years were older and had a higher prevalence of dyslipidemia and physical inactivity (Table 2).
Demographic, anthropometric, and cardiovascular risk data in the study subjects, stratified according to the smoking load quartiles.
| Smoking load (pack-years) | ||||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| ≤3 | 3–12 | 12.1–32 | >32 | |
| Age (years) | 40±11 | 40±12 | 48±9 | 57±9*,**,*** |
| Sex (Male/Female) | 8/16 | 12/13 | 10/14 | 5/17 |
| Weight (Kg) | 88.7±20.7 | 86.1±24.4 | 85.1±25.7 | 70.6±18.3 |
| Height (m) | 1.65±0.99 | 1.65±0.91 | 1.64±0.10 | 1.59±0.97 |
| BMI (Kg/m2) | 32.6±7.6 | 31.2±7.5 | 31.3±8.4 | 27.5±6 |
| Arterial hypertension | 25% | 20% | 20.8% | 18.2% |
| Diabetes mellitus | 12.5% | 4% | 8.3% | 31.8% |
| Dyslipidemia | 8.3% | 20% | 41.7% | 45.5%* |
| Obesity | 54.2% | 40% | 33.3% | 40.9% |
| Physical inactivity | 70.8% | 52% | 66.7% | 90.0%** |
BMI, body mass index.
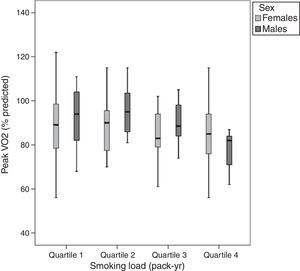
Despite the negative correlation between the SL and the peak V′O2, the peak V′O2 values were not statistically different among the SL quartiles (Fig. 2) when adjusted for age, sex and risk factors for cardiovascular disease.
Peak oxygen uptake at the end of cardiopulmonary exercise testing according to smoking load in pack-yr (quartile 1, ≤3; quartile 2, 3–12; quartile 3, 12.1–32; and quartile 4, >32). There were no significant differences among groups after multivariate analysis of variance adjusted by age, sex, arterial hypertension, diabetes, dyslipidemia, obesity, and physical inactivity.
In this study, we evaluated the influence of the SL on cardiopulmonary fitness in asymptomatic adult smokers and observed negative correlations between these variables. Despite these negative correlations, no dose–response relationship between the SL and the cardiorespiratory fitness was demonstrated.
We have also observed that the cardiopulmonary fitness as determined by the peak V′O2 was 12% lower than the expected in all four of the SL quartiles we studied, and about one-third (33.6%) of the subjects were exercise intolerant. In fact, our results reinforce the current literature. Misigoj-Durakovic et al.18 evaluated 350 Croatian Armed Forces members, of which 175 were smokers and 175 were non-smokers. The smokers were classified into three groups according to their SL (group 1, from 1 to 5 pack-years; group 2, from 5 to 10; and group 3, over 10 pack-years), and the non-smokers in three control groups. Cardiopulmonary fitness by treadmill CPET showed a significant reduction in the peak V′O2, even in smokers with an SL lower than five pack-years. As far as we know, very few studies have evaluated the negative effects of very low SL. As with the Croatian study, our results show that smoking has a negative effect on cardiopulmonary fitness in a non-dose dependent manner. The effect persisted at very low SLs even though the V′O2 was dose dependent and peaked, at an SL above three pack-years.
We showed that greater reductions in cardiopulmonary fitness in smokers with a higher SL are associated with age, dyslipidemia, and physical inactivity. According to Malta et al.,19 the frequency of intense smoking has a tendency to increase with age, increasing more than two fold between 18–24 and 55–64 years. Likewise, the cardiopulmonary and muscular fitness decreases as the individual ages.18 Smoking is also related to other cardiovascular risk factors. Studies have shown that heavy smokers present more comorbidities in comparison to light smokers.20 Heavy smokers have lower values in diffusing capacity, which explains the reduction in maximum exercise capacity.21 Nevertheless, it is possible that observational associations between heavy smoking and cardiovascular risks can be misinterpreted, since heavy smoking is related to sedentary behavior, clinical conditions and socioeconomic factors that cannot be entirely eliminated as potential confounders.22 Furlanetto et al.23 studied the level of physical activity in the daily life of 116 subjects, smokers and non-smokers, using a pedometer. The results have shown a significant reduction in the daily physical activity levels in adult smokers despite the absence of obstructive pulmonary disease. However, comparing the SL quartiles in our study, we found no difference in the negative effects of smoking in cardiopulmonary fitness on comparing subjects with an SL of 3 pack-years and subjects with an SL of more than 32 pack-years.
The SL did have a negative correlation with the main variables of the cardiopulmonary fitness in the CPET. The maximum heart rate showed very low average values (88% of the expected) despite the fact that the values for respiratory exchange ratio were compatible with maximum effort. A lower maximum heart rate in smokers during CPET was also observed by Unverdorben et al.24 Smoking alters the chronotropic response to exercise, increasing the risk of developing coronary artery disease and death.25 This deleterious effect on chronotropic response to exercise can lead to compromised cardiac output, to a reduction in transcutaneous oxygen tension, to a reduction in the anaerobic threshold, and to an increase in catecholaminerelease.26 In fact, a significant reduction in the peak V′O2 was even observed in SHS.25
Despite a reduction by 12% in the expected peak V′O2, the subjects of this study presented a normal anaerobic threshold (about 60% of the expected peak V′O2). In contrast to our results, Glaser et al.27 observed a significant reduction in the anaerobic threshold in smokers compared to non-smokers. However, physical inactivity affects the values of V′O2 and the anaerobic threshold, a confounder, which was not considered. It is well known that smokers present higher VE/V′CO2 ratios and a lower anaerobic threshold.27 This shows a reduction in ventilatory efficiency and certainly contributes to the overall reduction in the exercise capacity at higher but not at lower exercise intensity.
Some limitations of our study must be considered. Despite the correlations observed in the study, the cross-sectional design did not allow us to establish any relationship of cause and effect. We did not evaluate a control group of non-smokers, which might have changed the interpretation of the data. However, the predicted V′O2 values show that exercise capacity was clearly below normal. The convenient selection and the low sample size may have led us into an external validity issue regarding the SL sub-groups, and could explain the higher prevalence of comorbidities than in the general population. The lack of an objective control over the last cigarette smoked before CPET is another limitation of the present study. Smoking can cause acute effects in physiological responses to the CPET. However, all the CPETs were performed in the same cardiovascular clinic and the clinic stuff carefully advised our participants about avoiding exercise and smoking before the CPET. For this reason, we are confident we have been able to minimize this bias. Additionally, we did not evaluate the level of nicotine dependence. We recognize that this could be of great value since there are very few studies on the correlation of nicotine dependence and cardiopulmonary fitness. We should also state that the self-reported evaluations are subjective.
ConclusionBased on our findings, after adjusting the analysis for age and cardiovascular risk, there appears to be no dose–response relationship between SL and cardiopulmonary fitness in adult smokers with preserved pulmonary function. Therefore, smoking cessation might be useful as the primary strategy to prevent cardiopulmonary fitness decline in smokers, regardless of smoking load. Thus, even a very low dose of tobacco use must be avoided in preventive strategies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingThis study received financial support in the form of a research grant from the São Paulo Research Foundation (FAPESP), in the State of São Paulo, Brazil, grant no. 2011/07282-6.