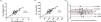The cardiopulmonary exercise test (CPET) is the gold standard for assessing aerobic fitness; however, it is expensive, not widely available, and requires specialized equipment and staff. The incremental shuttle walking test (ISWT) is an exercise field test used to evaluate exercise capacity and may be an alternative to CPET in patients with lymphangioleiomyomatosis (LAM).
ObjectiveTo investigate whether the ISWT can be used to assess maximal aerobic capacity in patients with LAM.
MethodsForty-five women were evaluated on two days, and they randomly performed the CPET and ISWT. The maximum oxygen uptake (peak VO2) was evaluated using gas analyzers in both tests. The carbon dioxide production (VCO2), respiratory exchange ratio (RER), and heart rate (HR) were compared during peak exercise. Pearson's correlation and Bland-Altman assessed the association and agreement, respectively. The intraclass correlation coefficient (ICC) was used to assess the reliability of the data.
ResultsAll patients (46.1 ± 10.2 years) presented similar peak VO2, RER, and peak HR during the CPET and ISWT (15.6 ± 4.6 vs. 15.7 ± 4.4 ml·kg−1·min−1; 1.15±0.09 vs. 1.17±0.12; and 142.2 ± 18.6 vs. 141.5 ± 22.2 bpm, respectively; p>0.05). A good linear correlation (r = 0.79; p<0.001) and ICC (0.86; 95%CI 0.74−0.93) were observed between the peak VO2 in both tests. Predictive peak VO2 equations based on the ISWT performance are also presented.
ConclusionOur results suggest that the ISWT can be used to assess maximal exercise performance in patients with LAM, and it is a valuable option to be used as an alternative to the CPET and predict maximal exercise capacity.
Lymphangioleiomyomatosis (LAM) is a rare neoplastic cystic lung disease that mainly affects women of reproductive age. It is characterized by the proliferation of abnormal smooth muscle-like LAM cells, resulting in vascular and airway obstruction and cyst formation.1 The main clinical characteristics of LAM include progressive dyspnea, pneumothorax, and chylothorax.2 The most common abnormalities observed in pulmonary function tests (PFTs) are an obstructive pattern, air trapping, and a reduction in the diffusion capacity of carbon monoxide (DLCO).1,3 Exercise limitation in patients with LAM is multifactorial4,5 and includes ventilatory limitation, cardiovascular dysfunction, gas exchange abnormalities, and muscle fatigue.4,6,7
Assessment of exercise capacity is a valuable clinical tool for evaluating the severity of a patient's functional impairment, providing maximal and submaximal variables through standardized exercise tests. The physiological rationale for performing these tests is to detect interesting variables that can be explained by the oxygen uptake and carbon dioxide output kinetic response to constant power exercise.8 The cardiopulmonary exercise test (CPET) is the gold standard for assessing exercise fitness; however, it is expensive and not widely available. Field-walking tests can be used as an alternative to assess exercise capacity because they are simple, inexpensive, and quick.9 The 6-min walking test (6MWT) is a submaximal exercise test widely used to assess functional exercise capacity in patients with moderate-to-severe pulmonary disease.9 However, previous studies have suggested that the 6MWT might not adequately assess patients with LAM because most of them present a ceiling effect.5,10,11 The incremental shuttle walking test (ISWT) has been used to quantify maximal exercise capacity in patients with chronic respiratory diseases, leading to physiological responses similar to the CPET.9 However, the cardiorespiratory and metabolic responses to the ISWT in patients with LAM remain little known.
In the present study, we hypothesized that patients with LAM who underwent the ISWT would have a similar cardiorespiratory response to CPET. In addition, we sought to determine the maximal exercise capacity considering the performance based on the ISWT in patients with LAM.
MethodsThis cross-sectional single-center study was conducted from September 2018 to March 2021 in a cohort of women with LAM from the interstitial lung disease (ILD) outpatient clinic at a tertiary university hospital. The diagnosis of LAM was based on the current guidelines,2,12 which included PFTs, computed tomography scans, serum analysis, and, if necessary, lung biopsy. The Ethics Research Hospital Committee (90,196,617.1.0000.0068) approved the protocol. All participants signed the informed consent form. The patients were clinically stable (no exacerbation for the last six weeks),12 and they maintained peripheral resting oxygen saturation (SpO2) ≥90% in room air. The exclusion criteria were supplemental oxygen use, other chronic respiratory diseases, uncontrolled heart disease, pregnancy, or any limiting condition that could interfere with the tests.
Experimental designPatients were evaluated during two nonconsecutive visits (one week apart). During the 1st visit, clinical and anthropometric data were obtained, and the participants performed the PFT and 6MWT. After at least 30 min of recovery, patients were randomly assigned (http://www.randomization.com) to the CPET or ISWT by an investigator not involved in the assessments. The remaining test was performed in the 2nd visit.
AssessmentsPFTsSpirometry and body plethysmography (Bodystik Geratherm Respiratory GmbH, Bad Kissingen, Germany) were performed to obtain the lung volumes (forced expiratory volume in 1 second [FEV1] and residual volume [RV]), capacity (inspiratory capacity [IC], forced vital capacity [FVC], functional residual capacity [FRC], and total lung capacity [TLC]), and diffusion capacity for carbon monoxide (DLco). The predicted values were based on those of the Brazilian population.13-15 Obstructive patterns, air trapping, and reduced DLco were defined according to the ATS/ERS.16
Peripheral muscle strengththe quadriceps strength was measured with a load cell integrated into a circuit in a chair fixed on a wooden plank. The load cell was calibrated and attached to the chair's base with an inextensible strap. One side of the strap was fixed to the ankle, and the other side was fixed to the load cell, keeping the knee flexed at 90° During the test, the patients were asked to cross their arms and extend their dominant knee.17 Three consecutive 5-s efforts were made at 30-s intervals, with verbal encouragement from the investigator. The maximum value was used for the analysis.18
Dyspnea and leg fatigue perceptionthe modified Borg scale (ranging from 0 to 10; 0=absence of symptoms, 10= the worst perception of dyspnea/leg fatigue) was used to assess the intensity of dyspnea and leg fatigue during exercise.19
Exercise testsCPET: was performed using an electrical cycle ergometer (Corival, Lode B.V.; Medical Technology, the Netherlands) digitally equipped with an exercise evaluation system (CPX System; CareFusion Corporation, Germany), according to the ERS recommendations.20 Peripheral oxygen saturation (SpO2) and electrocardiography were continuously monitored during the tests. The work rate (W), oxygen consumption (VO2), minute ventilation (VE), carbon dioxide production (VCO2), respiratory exchange rate (RER), and heart rate (HR) were recorded. The delta (Δ, final–initial) heart rate (Δ, HR) was obtained by subtracting the peak HR from the basal HR. Blood pressure (BP), leg discomfort, and dyspnea19 were also monitored. Patients performed a symptom-limited CPET, consisting of 2-min of rest, 2-min of warm-up (unloaded pedaling), and a ramp work period (10–15 W), taking into account the patient's normal daily activity level. The predicted maximum VO2 was obtained from a Brazilian population.21 The MVV was estimated by multiplying the absolute FEV1 (in liters) by 40, and then the ventilatory reserve was calculated by subtracting the maximal ventilation value obtained at the peak of exercise (VE, in liters) from the MVV, divided by the MVV, and multiplied by 100. Ventilatory limitation was considered if the ventilatory reserve was <20%.22 The cardiac reserve was calculated as the difference between the predicted HR and the HR achieved during CPET, divided by HR achieved and multiplied by 100. Cardiovascular limitations were considered if the cardiac reserve was <10%.22 The patient was classified as desaturators if presenting a drop in SpO2 ≥4%23 during any exercise test.
ISWT: was conducted on an empty 10-m corridor, and the same variables evaluated in the CPET were assessed using a portable Oxycon mobile device (Carefusion, Houten, The Netherlands). The patient's walking speed was determined using a standardized audio signal (beep), and performed as previously described.24,25 Tests were discontinued if SpO2 decreased below 80%.9 Before and after the test, the HR, BP, the minimum SpO2 maintained for at least 10 s, dyspnea, and fatigue symptoms were assessed.
6MWT: the patient was asked to walk as far as possible along a 30-m corridor for 6-min, and the 6-min walk distance (6MWD) was obtained.25 The predicted values for the distance walked were based on those of the Brazilian population.26 Tests were discontinued if SpO2 decreased below 80%.9 Before and after the test, the HR, BP, the minimum SpO2 maintained for at least 10 s, dyspnea, and fatigue symptoms were assessed.
Quality of lifeThe Medical Outcomes Study Short-Form 36 (SF-36)27 has been used to evaluate the health-related quality of life in patients with chronic respiratory disease. The SF-36 has eight domains: physical functioning, role physical, pain, general health, vitality, role social, role emotional, and mental health (the score ranges from 0 to 100, the highest score=a better health status).
Safety of the ISWTSafety was defined as the number and severity of reported or observed adverse events during the ISWT. Oxygen desaturation was considered when SpO2 values were reduced by at least 4% during exercise tests. In addition, the presence of oxygen desaturation, chest pain, pre-syncope, or syncope were considered to evaluate the safety of the ISWT.28
Statistical analysisData are reported as the mean±standard deviation or median (25–75% interquartile range), according to normality distribution. The Kolmogorov–Smirnov test was used to assess the data normality. The data comparison was assessed by paired t-test and chi-square to compare categorical data. The association between the CPET and ISWT parameters was assessed by Pearson correlation (r) (weak [0.10–0.29], moderate [0.30–0.49], strong [0.50–1.00]).29 The intraclass correlation coefficient (ICC) assessed the reliability ((poor [<0.49], moderate [0.50≥ICC<0.74], good [0.75≥ICC≤ 0.89], reliability excellent [≥0.90]).30 Bland-Altman evaluated the agreement between tests. Finally, a stepwise multiple linear regression analysis was performed to predict VO2, involving variables with a p-value<0.2 obtained from a linear correlation test. The level of significance was set at 5%. Data were analyzed using Sigma Stat version 3.5 (Systat Software, Inc., San Jose, CA).
ResultsSixty women with LAM were invited to participate in the study. Fifteen patients declined because they could not return for the 2nd visit (Fig. 1). Therefore, 45 women were included, and their clinical, anthropometric, and functional data are presented in Table 1. The patients were of the median age (45 [38−54] years), had good quadriceps muscle strength (78% of predicted [67–96%]), and quality of life (65 score [55−81]). In addition, the obstructive airway pattern, air trapping, and reduced DLCO were found in 60%, 57%, and 15% of the patients, respectively.
Baseline demographic, quality of life, clinical, and functional characteristics.
Legend: Data are presented as the median and interquartile range (25%−75%). BMI, body mass; *2 patients (4.4%) did not undergo echocardiogram; SF36, Short Form 36 Health Survey; 6MWD, six-minute walking distance; ISWT, incremental shuttle walking test; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; DLco, diffusion of carbon monoxide; RV, residual volume; TLC, total lung capacity.
On average, patients had good exercise capacity assessed during the 6MWT and ISWT, 90% (83–100%) and 78% (67–96%) of the predicted values, respectively. Thirty-nine patients (86%) reached the peak VO2 below 84% of the predicted value during the CPET. Twenty-one patients (46.6%) were unable to reach the speed imposed by the beep during the ISWT, and 24 (53.3%) were limited by symptoms (18, dyspnea and 6, fatigue).
In the comparison between the CPET and ISWT, there were no differences between the variables related to cardiopulmonary responses, except for a greater leg fatigue perception in the CPET (Table 2). Thirteen (29%) patients presented ventilatory and 22 (49%) cardiovascular limitations during the CPET and ISWT. Analyzing only patients with cardiac and ventilatory limitations, a difference was observed between peak VO2 during ISWT (1179±307 vs 948±280 ml.min−1, respectively; p<0.005). Interestingly, it was also observed that patients with cardiac limitations presented higher VO2 compared with those with ventilatory limitations during CPET (1227±278 vs 930±272 ml.min−1, respectively; p<0.005).
Comparison of variables obtained in the cardiopulmonary exercise test and in the incremental shuttle walking test.
Legend: Data are presented as mean (SD) or median (interquartile range 25%−75%). The patient was considered a desaturator if presented a drop in oxygenation was ≥ 4% during any exercise test. The cardiac and respiratory reserves are described in the Methods section. CPET, cardiopulmonary exercise test; HR, heart rate; ISWT: incremental shuttle walking test; bpm, beats per minute, VO2 oxygen peak uptake; ml/min, milliliters per minute; kg, kilograms; RER, respiratory exchange ratio; D, dyspnea; F, fatigue.
A strong linear correlation was also observed between the peak VO2 obtained during the CPET and ISWT in ml*kg−1*min−1 and ml*min−1 (r = 0.78 and r = 0.75, p<0.001; Fig. 2A-B). The ICC showed a good agreement (0.88; 95% CI, 0.78−0.93). The Bland-Altman plot showed that the peak VO2 has an average difference of 0.07 ml*kg−1*min−1 between the CPET and ISWT and most patients (66%) presented a small limit of agreement (−2.8 to 2.8 ml*kg−1*min−1; 1SD) (Fig. 2C).
(A, B, C). Linear correlation of the obtained peak VO2 between the CPET and the ISWT in ml*Kg−1*min−1 (A) and ml*min−1 (B); Pearson correlation test was used in both analyses. Bland Altman Graphic (C) representation between the differences in VO2 peak values in CPET and ISWT. The solid line corresponds to the difference between the means of the upper and lower limits of agreement. Abbreviations CPET, cardiopulmonary exercise test; ISWT, incremental shuttle walking test; SD, standard deviation.
The dependent variables were the peak VO2 in kg*ml−1*min−1 and ml*min1 obtained during the CPET and ISWT. Independent variables included airway obstruction (FEV1), ISWT duration, gas exchange (DLco), body weight, and changes in the heart rate (ΔHR). The derived prediction equations are as follows:
(Body weight, in kg; FEV1, in liters; ISWT duration, in seconds)
(DLco, in absolute values; ISWT duration, in seconds; Δ, HR, in beats per min)
(Body weight, in kg; FEV1, in liters; ISWT duration, in seconds)
(ISWT duration, in seconds; DLco, in absolute values; (Δ, HR, in beats per min)
Seventeen (38%) patients were classified as desaturators during the CPET and 42 (93%) patients during the ISWT. Despite the high frequency of oxygen desaturation during the ISWT, no adverse events were observed or reported. In addition, the perception of dyspnea and fatigue 4 (3 − 7) and 3 (2 − 6), respectively, reinforced good tolerance during the ISWT.
DiscussionTo the best of our knowledge, this is the first study to evaluate the use of field tests to assess maximal exercise performance in patients with LAM. Our results show that: (i) the ISWT elicited similar peak VO2, HR, RER, and dyspnea perception as the CPET; (ii) the ISWT elicited a greater oxygen desaturation than the CPET; and (iii) the peak VO2 in the CPET and ISWT could be predicted based on lung function data, body weight, and ISWT performance; (iv) the ISWT was considered safe and patients with LAM presented a good tolerance to such tests.
Women with LAM present exercise limitations that are sometimes not linearly supported by the degree of airway obstruction.4 The 6MWD has a good linear relationship with maximal exercise capacity (peak VO2) in several respiratory diseases9; however, most patients with LAM reach the ceiling effect.31 Our patients reached, on average, 90% of the predicted distance in the 6MWT, reinforcing the findings of the previous studies,4,11 which demonstrated that this test could not be properly used to evaluate exercise capacity in this population.
Meanwhile, our results showed that patients with LAM had similar responses during the CPET and ISWT, according to the peak VO2 and maximal HR. Previous studies in chronic obstructive pulmonary disease patients observed similar findings demonstrating similar maximal exercise performance during the CPET and ISWT.32-34 In addition, we observed a strong linear correlation between the peak VO2 obtained in the CPET and ISWT (r = 0.79). Interestingly, Singh et al. (2018)35 evaluated 27 patients with ILDs and observed a positive linear association between the peak VO2 in the CPET and the distance assessed in the ISWT (r = 0.79; p<0.001). It is important to emphasize that we evaluated a more significant number of patients with a rare disease, such as LAM. In addition, we evaluated the linear association between the peak VO2 during the CPET and ISWT, which is unusual because it requires a portable oxygen analyzer.
At last, we observed that patients with cardiac presented higher peak VO2 during ISWT and CPET than those with ventilatory limitations. These results add value to our results, as they show that both tests work similarly regardless of the exercise limitation. Taken together, our findings suggest that ISWT can be an alternative to assess physical performance in clinically stable women with LAM.
We also presented equations to establish the variables associated with predicting the peak VO2 during either the CPET or ISWT in women with LAM. Our results show that approximately 70% of the peak VO2 (either in ml*min−1 or ml*kg−1*min−1) during the CPET was explained by functional impairment (FEV1), body weight, and ISWT duration. These results are very useful in clinical practice because they allow the prediction of VO2 with a high chance of success based on anthropometrical and functional data after submitting a patient with LAM to the ISWT. In addition, the peak VO2 obtained during the ISWT was explained 60% by the DLco, ISWT duration, and the increase in ∆HR. Patients' oxygen desaturation during exercise is common in individuals with LAM36 and has been associated with airway obstruction, hyperinflation, and reduced DLCO in patients with LAM.4 Interestingly, our patients presented a higher frequency of oxygen desaturation during the ISWT than during the CPET. We hypothesize that this occurred because a large muscles group is recruited during walking and increases oxygen consumption more than the cycle ergometer when the quadriceps is the most demanded muscle group and leads to increased local muscle fatigue perception (Table 2). However, no adverse events were reported or observed during the ISWT, and the fatigue perception reinforced a good tolerance to the test. These results suggest that performing the ISWT is safe in this population. However, patients presented greater desaturation during the ISWT (Table 1), which can be explained by the recruitment of a greater muscle mass during walking in the ISWT than in the CPET performed on a cycle ergometer. Although this population can be considered one of the largest ever studied, further studies with larger sample sizes are necessary to evaluate patient safety during field tests in this population. Moreover, our results cannot be extrapolated to all exercise tests.
Our study had several limitations. First, the study was performed in a single center; however, our center is the referral center for LAM in Brazil, and we receive patients from several regions with different disease severities. In addition, 45 participants can be considered a significant sample size due to the rarity of the disease (one in a million adult women).37 Second, we excluded patients under long-term oxygen therapy, and our results cannot be extrapolated to this subgroup. Third, the CPET was performed in a cycle ergometer, which may explain the greater leg fatigue observed in the CPET than in the ISWT. However, the cycle ergometer is the most common modality used in the laboratory-based CPET in patients with chronic respiratory diseases.38 In addition, the maximal predicted peak VO2 values for the Brazilian population were based on a cycle ergometer.39 Finally, CPET in a cycle ergometer is less prone to movement artifacts, allowing the assessment of blood pressure, arrhythmias, myocardial ischemia, and other abnormalities.8
Our results showed that the ISWT induced a similar response to the CPET in women with LAM. We also presented predictive peak VO2 equations based on ISWT performance. Finally, the ISWT appears to be safe in LAM since no adverse events were observed.
Funding informationThe study was supported by the grants 312,279/2018–3 from the Ministerio da Ciencia, Tecnologia e Inovação - Conselho Nacional de Pesquisa (CNPq) and grants 2018/17,788–3 from Fundação de Amparo à Pesquisa de São Paulo (FAPESP).