Malignant pleural effusion (MPE) is a common complication in advanced stages of malignancy and is associated with poor prognosis. Non-expandable lung (NEL) often occurs and its presence influences the MPE approach. Our main objective was to assess risk factors for malignant NEL.
MethodsPatients diagnosed with pathologically confirmed MPE between January 2012 and December 2018 in our institution were retrospectively analyzed. Demographic and clinical data of patients were reviewed and compared according to the presence or absence of NEL. A univariate and multivariate binary logistic regression analysis were used to determine predictors of the development of NEL.
ResultsOf 365 patients included, 68 (18.6%) had NEL. After multivariate analysis, we found that loculated MPE (OR 8.63, 95%CI 4.30-17.33, p<0.001), complete hemithorax opacification (OR 2.81, 95%CI 1.17-6.76, p<0.021), lung cancer (OR 2.09, 95%CI 1.01-4.31, p=0.047) and higher effusion-serum LDH ratio (OR 1.09, 95%CI 1.00-1.17, p=0.039) were independent predictors of malignant NEL. There were no significant differences compared with expandable lung group regarding time from primary malignancy diagnosis to MPE diagnosis (3.0, IQR 0.0-75.8 vs 2.0, IQR 0.0-75.5 weeks, p=0.942) or MPE symptoms onset to MPE diagnosis (4.0, IQR 1.0-9.0 vs 3.0, IQR 1.0-9.0 weeks, p=0.497). Patients with NEL had a higher number of therapeutic pleural drainages (3.0, IQR 2.0-6.0 vs 2.0, IQR 1.0-3.0; p<0.001) and longer hospital stay (32.5, IQR 15.5-46.3 vs 21.0, IQR 11.0-36.0, p=0.007), measured in hospitalization days until the end of life, than patients with expandable lung. The rate of recurrence of pleural effusion was not significantly different between groups (p=0.291). Overall survival (OS) was 3.0 (95%CI, 2.3-3.7) months, regardless of lung expandability (p=0.923).
ConclusionLoculated MPE, complete hemithorax opacification, lung cancer and a higher effusion-serum LDH ratio were found to be independent predictors for NEL. These patients underwent thoracocenteses more frequently and had longer hospitalization days, although without significant impact in the OS.
Malignant pleural effusion (MPE) is a common complication in advanced stages of malignancy and affects approximately 15% of oncological patients.1,2 This condition is associated with poor prognosis, with an overall survival (OS) from 3 to 12 months.3,4 Despite the advances in cancer treatments, MPE management remains palliative and focuses on patient's symptoms relief.4-6 However, there are factors to be taken into account when choosing a treatment, including lung expandability.7
Non-expandable lung (NEL) due to lung entrapment is often found in MPE patients, and may be caused by endobronchial obstruction or malignant involvement of visceral pleura,8 which prevents visceral and parietal pleura apposition following pleural fluid drainage.9 The presence of a hydropneumothorax after thoracocentesis or the inability to completely drain the pleural effusion due to excessive cough or central chest pain during aspiration strongly suggest the presence of lung entrapment. As the lung cannot expand, the pleural pressure becomes more negative, which justifies the continuous transudation of fluid from the parietal pleural capillaries and frequent recurrence of pleural effusion, with a significant impact on the quality of life of these patients. Unfamiliarity with this entity can lead to unnecessary and ineffective interventions, with significant morbidity.10 Intermittent pleural drainage or indwelling pleural catheter (IPC) have been reported to be a better option for the management of malignant lung entrapment.5,11
Thus, early detection and management of MPE should improve outcomes, by decreasing the occurrence of lung entrapment and allowing more patients to benefit from definitive treatment with pleurodesis. Looking for a more patient-centered approach to MPE, this study aimed to identify possible predictors of lung entrapment by the retrospective analysis of patients diagnosed with MPE during a period of six years in our institution.
Material and methodsPatientsPatients diagnosed with MPE, defined as malignant cells present in cytological pleural fluid analysis or in histopathological examination of pleural specimens, between January 2012 and December 2018 and followed-up in Centro Hospitalar Universitário de São João (CHUSJ), a Portuguese university hospital, were retrospectively analysed.
Demographic and clinical data at the time of MPE diagnosis were collected, including: (i) tumour origin, histological type and systemic treatments; (ii) biochemical, differential cell count and cytological results of the pleural fluid; (iii) pleural histopathology results; (iv) peripheral full blood count and biochemical analysis. All performed procedures, either for diagnosis (thoracocentesis, thoracoscopic or percutaneous pleural biopsy) or for treatment (serial thoracocentesis, talc poudrage or slurry pleurodesis, or IPC placement), related complications, as well as radiological follow-up and death, were recorded.
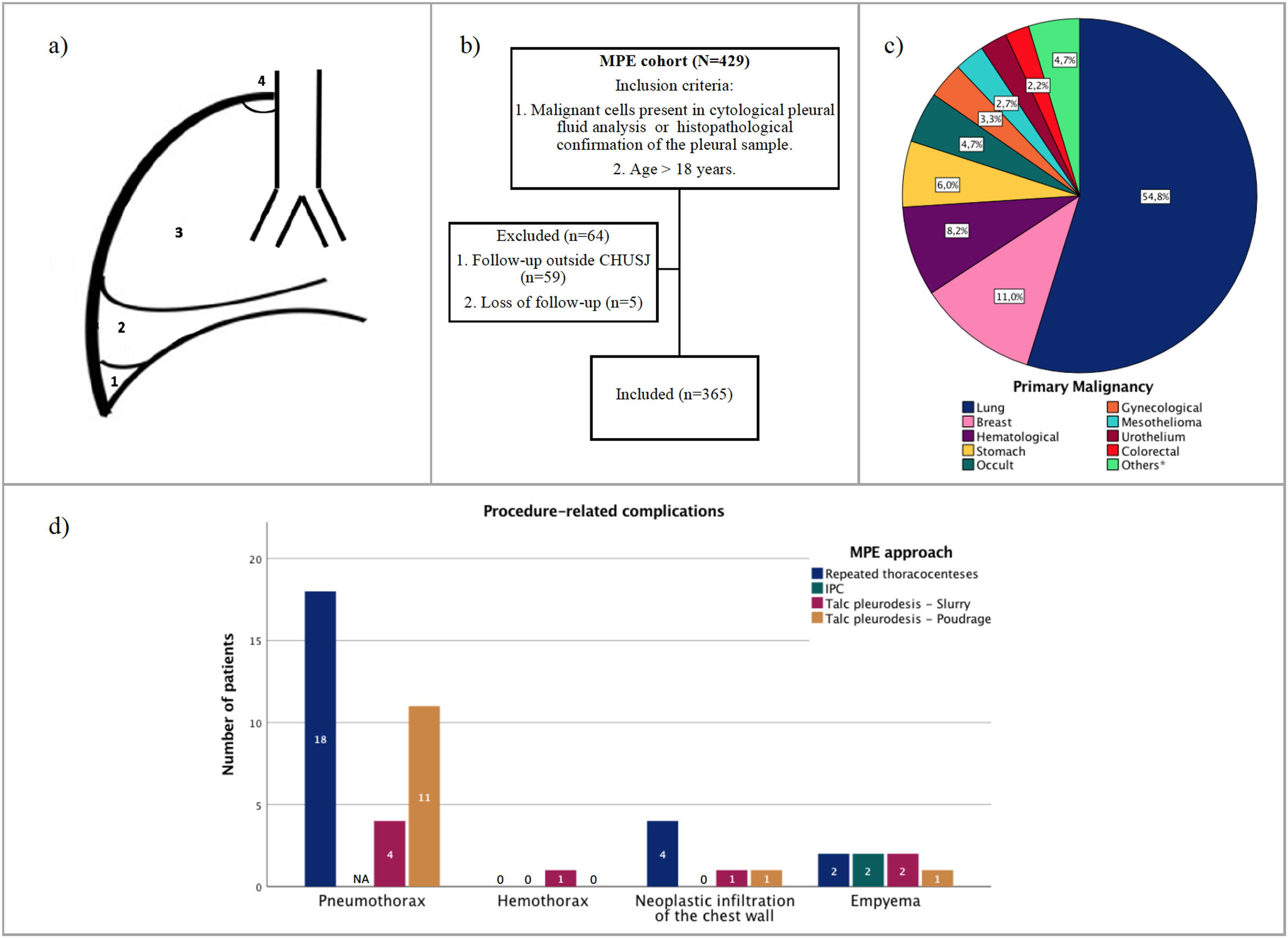
Radiological assessmentRadiological assessment was based on posteroanterior chest X-ray (CXR) performed at the time when pleural effusion was detected. In order to measure more objectively the pleural effusion progression, we created a classification system (Fig 1a). A small effusion was defined when pleural fluid line was detected under the hemidiaphragm, blunting the costophrenic angle. Moderate effusion was considered when fluid line was detected between the aforementioned level and slightly above the hemidiaphragm, whereas when exceeding this level, a large pleural effusion was considered or complete hemithorax opacification.
(a) Pleural effusion size classification- (1): Small pleural effusion; (2): Moderate pleural effusion; (3): Large pleural effusion; (4) Complete hemithorax opacification; (b) Study design; (c) Primary malignancies frequencies (*Esophageal, pancreas, bile ducts, thyroid, skin, oropharynx); (d) Procedure-related complications. NA: Not applicable.
A loculated MPE was defined based on the presence of ≥1 fixed pockets of fluid observed on thoracic ultrasound (TUS) or if described on chest computed tomography (CT) scans.
Pleural proceduresAll patients underwent initially a pleural fluid aspiration for diagnosis, in which 20-50 ml of pleural fluid is collected using a small-bore needle and a syringe. A commercial kit (Oasis Dry Suction Water Seal Chest Drain, Getinge, Sweden) is used to drain the pleural fluid in order to relieve patient's symptoms and to assess lung expandability. Pleural manometry is not applied routinely. The drained volume is guided by patients’ symptoms with a maximum amount of 1500-2000 ml. TUS guidance was used depending on the pulmonologist experience and equipment availability; this started to be increasingly used from 2014 onwards in our department. Percutaneous pleural biopsy was performed, either by pulmonologists using Cope needle, as originally described12 or, less frequently, by radiologists using CT or ultrasound-guided core needle biopsy. When the obtained samples were inconclusive for malignancy, a diagnostic single-port thoracoscopy using the rigid thoracoscope (Karl Storz, Germany) for pleural sampling was done. Pleurodesis was performed either by talc slurry administration via chest tube, or talc poudrage during thoracoscopy, using sterile asbestos-free talc (Steritalc®; Novatech, France). In symptomatic patients with recurrent MPE, NEL, and/or unsuccessful pleurodesis, an indwelling pleural catheter (Rocket® IPC) was placed, except in cases of patients’ refusal or contraindications.13
OutcomesThe primary outcome of this study was to evaluate factors associated with NEL, defined as incomplete lung expansion with <50% pleural apposition in CXR following the first pleural tap.14 In addition, secondary analyses included the time from primary malignancy diagnosis to MPE diagnosis (through pleural tap/biopsy), the time from the first radiological evidence of pleural effusion to MPE diagnosis, the time from the symptoms onset (dyspnea, dry cough or chest pain) to MPE diagnosis, and the duration of hospitalization, defined as the cumulative number of nights spent in the hospital after MPE diagnosis. We also evaluated recurrence of pleural effusion after pleurodesis, and complications associated with different treatment approaches to MPE. Finally, we assessed the OS, calculated from the MPE pathological diagnosis until death or loss of follow-up.
StatisticsCategorical variables are presented as frequencies and percentages, and continuous variables as means and standard deviations (SD), or medians and interquartile ranges (IQR) for variables with skewed distribution. Normal distribution was tested using skewness and kurtosis. Chi-square and Fisher's exact tests were used to compare categorical variables. Independent-samples t-test or Mann-Whitney U test was used to evaluate differences in continuous variables with normal distribution or non-normal distribution, respectively.
Univariate and multivariate logistic regression analyses were performed for crude and adjusted odds ratio (OR) calculation, to determine predictors of lung entrapment. Variables with a p<0.200 in the univariate analysis were included in the multivariate regression, and independent predictors were identified after a backward stepwise selection. OS, with respective 95% confidence intervals (95% CI), was estimated by the Kaplan-Meier method and differences between the curves were analysed using the log-rank test. The p-value considered for statistical significance was 0.05. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, IBM Corp, Chicago, IL, USA) software, version 27.0.
Ethical approvalThe registration protocol is in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics and Health Committee of CHUSJ (approval number 59-21).
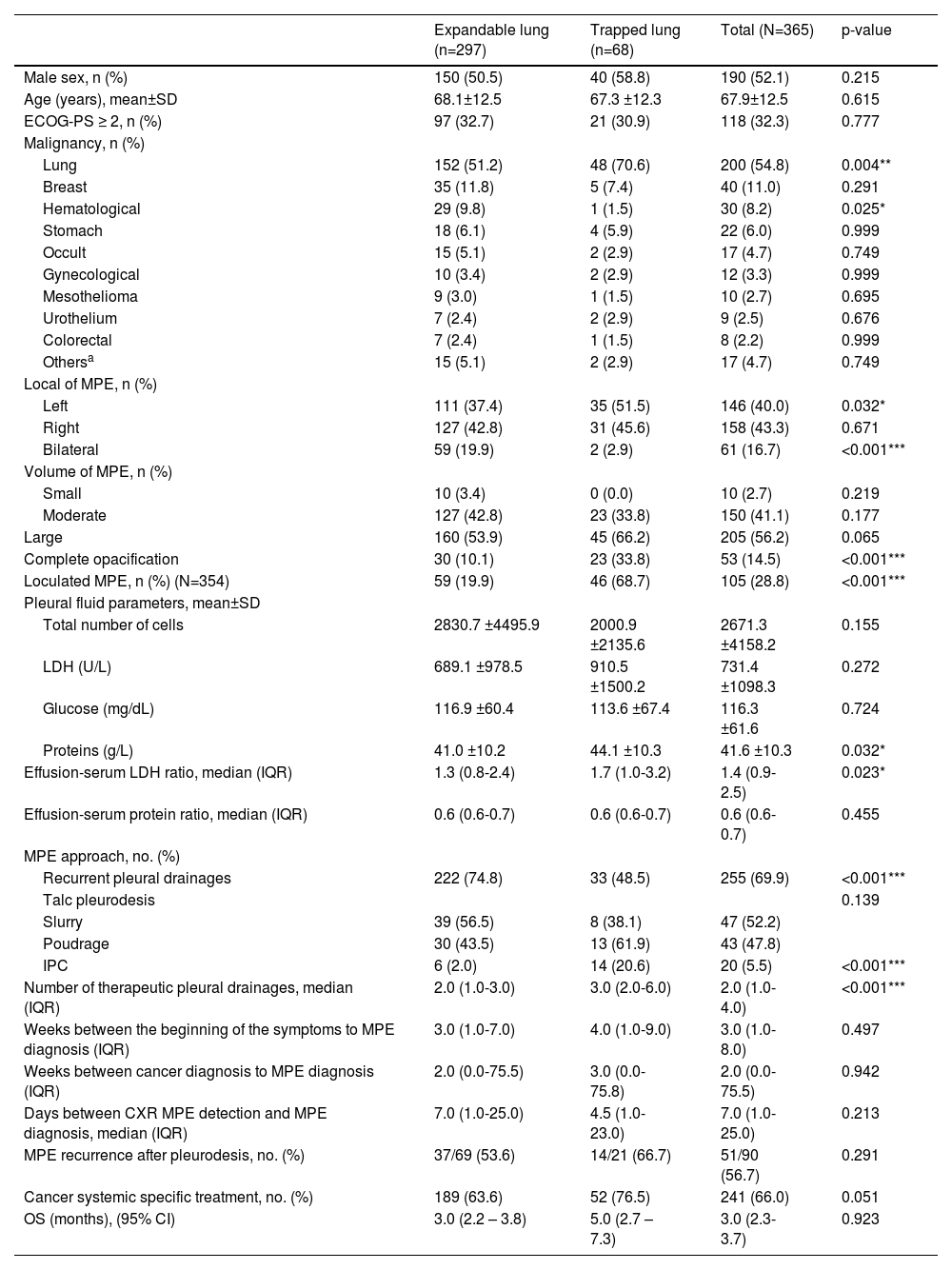
ResultsBaseline featuresOf the 429 patients diagnosed with MPE during the 6 year-period under analysis, 365 patients were included in the study (Fig 1b) and their baseline characteristics are presented in Table 1. The mean age was 67.9±12.5 years, 52.1% were male and the most common primary malignancies (Fig 1c) were lung cancer (54.8%), breast cancer (11.0%) and haematological malignancy (8.2%). The majority of patients presented a unilateral (83.3%) and a large volume (56.2%) pleural effusion. A loculated effusion was found in 105 (28.8%) patients. Nearly one-third (32.3%) had an ECOG-PS score ≥2 and 241 (66%) patients received cancer systemic specific treatment following MPE diagnosis.
Baseline characteristics of patients with malignant pleural effusion.
| Expandable lung (n=297) | Trapped lung (n=68) | Total (N=365) | p-value | |
|---|---|---|---|---|
| Male sex, n (%) | 150 (50.5) | 40 (58.8) | 190 (52.1) | 0.215 |
| Age (years), mean±SD | 68.1±12.5 | 67.3 ±12.3 | 67.9±12.5 | 0.615 |
| ECOG-PS ≥ 2, n (%) | 97 (32.7) | 21 (30.9) | 118 (32.3) | 0.777 |
| Malignancy, n (%) | ||||
| Lung | 152 (51.2) | 48 (70.6) | 200 (54.8) | 0.004** |
| Breast | 35 (11.8) | 5 (7.4) | 40 (11.0) | 0.291 |
| Hematological | 29 (9.8) | 1 (1.5) | 30 (8.2) | 0.025* |
| Stomach | 18 (6.1) | 4 (5.9) | 22 (6.0) | 0.999 |
| Occult | 15 (5.1) | 2 (2.9) | 17 (4.7) | 0.749 |
| Gynecological | 10 (3.4) | 2 (2.9) | 12 (3.3) | 0.999 |
| Mesothelioma | 9 (3.0) | 1 (1.5) | 10 (2.7) | 0.695 |
| Urothelium | 7 (2.4) | 2 (2.9) | 9 (2.5) | 0.676 |
| Colorectal | 7 (2.4) | 1 (1.5) | 8 (2.2) | 0.999 |
| Othersa | 15 (5.1) | 2 (2.9) | 17 (4.7) | 0.749 |
| Local of MPE, n (%) | ||||
| Left | 111 (37.4) | 35 (51.5) | 146 (40.0) | 0.032* |
| Right | 127 (42.8) | 31 (45.6) | 158 (43.3) | 0.671 |
| Bilateral | 59 (19.9) | 2 (2.9) | 61 (16.7) | <0.001*** |
| Volume of MPE, n (%) | ||||
| Small | 10 (3.4) | 0 (0.0) | 10 (2.7) | 0.219 |
| Moderate | 127 (42.8) | 23 (33.8) | 150 (41.1) | 0.177 |
| Large | 160 (53.9) | 45 (66.2) | 205 (56.2) | 0.065 |
| Complete opacification | 30 (10.1) | 23 (33.8) | 53 (14.5) | <0.001*** |
| Loculated MPE, n (%) (N=354) | 59 (19.9) | 46 (68.7) | 105 (28.8) | <0.001*** |
| Pleural fluid parameters, mean±SD | ||||
| Total number of cells | 2830.7 ±4495.9 | 2000.9 ±2135.6 | 2671.3 ±4158.2 | 0.155 |
| LDH (U/L) | 689.1 ±978.5 | 910.5 ±1500.2 | 731.4 ±1098.3 | 0.272 |
| Glucose (mg/dL) | 116.9 ±60.4 | 113.6 ±67.4 | 116.3 ±61.6 | 0.724 |
| Proteins (g/L) | 41.0 ±10.2 | 44.1 ±10.3 | 41.6 ±10.3 | 0.032* |
| Effusion-serum LDH ratio, median (IQR) | 1.3 (0.8-2.4) | 1.7 (1.0-3.2) | 1.4 (0.9-2.5) | 0.023* |
| Effusion-serum protein ratio, median (IQR) | 0.6 (0.6-0.7) | 0.6 (0.6-0.7) | 0.6 (0.6-0.7) | 0.455 |
| MPE approach, no. (%) | ||||
| Recurrent pleural drainages | 222 (74.8) | 33 (48.5) | 255 (69.9) | <0.001*** |
| Talc pleurodesis | 0.139 | |||
| Slurry | 39 (56.5) | 8 (38.1) | 47 (52.2) | |
| Poudrage | 30 (43.5) | 13 (61.9) | 43 (47.8) | |
| IPC | 6 (2.0) | 14 (20.6) | 20 (5.5) | <0.001*** |
| Number of therapeutic pleural drainages, median (IQR) | 2.0 (1.0-3.0) | 3.0 (2.0-6.0) | 2.0 (1.0-4.0) | <0.001*** |
| Weeks between the beginning of the symptoms to MPE diagnosis (IQR) | 3.0 (1.0-7.0) | 4.0 (1.0-9.0) | 3.0 (1.0-8.0) | 0.497 |
| Weeks between cancer diagnosis to MPE diagnosis (IQR) | 2.0 (0.0-75.5) | 3.0 (0.0-75.8) | 2.0 (0.0-75.5) | 0.942 |
| Days between CXR MPE detection and MPE diagnosis, median (IQR) | 7.0 (1.0-25.0) | 4.5 (1.0-23.0) | 7.0 (1.0-25.0) | 0.213 |
| MPE recurrence after pleurodesis, no. (%) | 37/69 (53.6) | 14/21 (66.7) | 51/90 (56.7) | 0.291 |
| Cancer systemic specific treatment, no. (%) | 189 (63.6) | 52 (76.5) | 241 (66.0) | 0.051 |
| OS (months), (95% CI) | 3.0 (2.2 – 3.8) | 5.0 (2.7 – 7.3) | 3.0 (2.3-3.7) | 0.923 |
Legend: CI, confidence interval; CXR, chest X-ray; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; IPC, Indwell Pleural Catheter; IQR, Interquartile range; LDH, lactate dehydrogenase; MPE, Malignant pleural effusion; OS, Overall survival; SD, standard deviation.
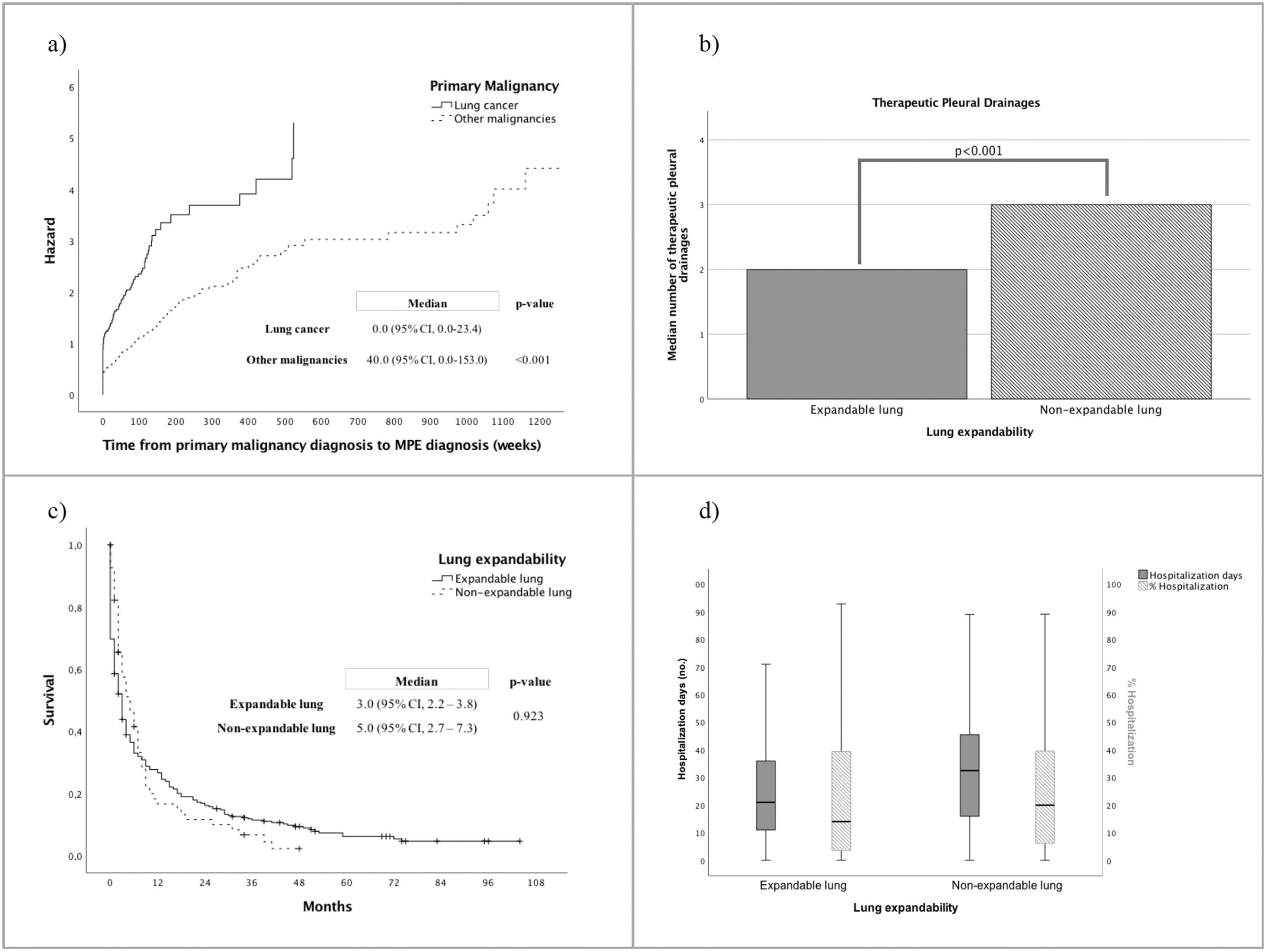
The median time from primary malignancy diagnosis to MPE detection was 2.0 (IQR, 0.0-75.5) weeks, which was significantly shorter in the group of patients with lung cancer (0.0, 95%CI 0.0-153.0 weeks) compared to other malignancies (40.0, 95%CI 0.0-23.3 weeks, p<0.001) (Fig 2a). The median time observed from symptoms onset to MPE diagnosis was 3.0 (IQR, 1.0-8.0) weeks.
(a) Time from cancer diagnosis to MPE diagnosis according to primary malignancy (weeks); (b) Median number of therapeutic pleural drainages according to lung expandability; (c) Overall survival according to lung expandability (months); (d) Number of days of hospitalization and ratio between number of hospitalization days per number of days alive since the MPE diagnosis (%), according to lung expandability.
NEL was more frequently found in lung cancer patients (70.6%), with loculated MPE (68.7%) and when large volume pleural effusion causing complete opacification of a hemithorax (33.8%), also known as unilateral lung white-out (Table 1). Fifty-nine out of 61 patients with bilateral pleural effusion had expandable lungs. Moreover, patients with lung entrapment had higher protein levels of the pleural fluid (mean 44.1±10.3 vs. 41.0±10.2; p=0.032), and/or higher effusion-serum LDH ratio (median 1.7 [IQR 1.0-3.2] vs. 1.3 [IQR 0.8-2.4], p=0.023) than expandable lungs. No additional pleural effusion analytical parameters were linked to NEL. The time between radiological pleural effusion detection and MPE diagnosis did not have significant impact on the development of NEL.
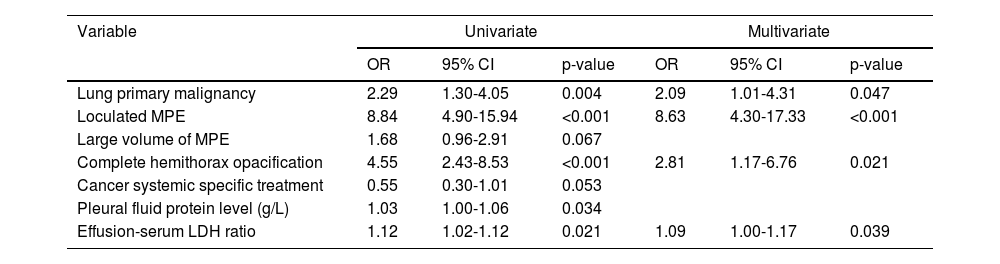
Univariate and multivariate analyses for predictors of lung entrapment are presented in Table 2. Loculated pleural MPE (OR 8.63; 95%CI:4.30-17.33, p<0.001), complete hemithorax opacification (OR 2.81; 95%CI:1.17-6.76, p=0.021), lung cancer (OR 2.09, 95%CI:1.01-4.31, p=0.047), and higher effusion-serum LDH ratio (OR 1.09, 95%CI:1.00-1.17, p=0.039) were independent predictors for the occurrence of lung entrapment.
Univariate and multivariate analyses for predictors of lung entrapment.
Legend: CI, confidence interval; LDH, lactate dehydrogenase; MPE, Malignant pleural effusion; OR, Odds ratio.
Serial thoracocenteses was the most frequent approach to MPE (n=255; 69.9%), with higher number of recurrent pleural drainages required in patients presenting lung entrapment, than in cases of expandable lung (3.0, IQR 2.0-6.0 vs. 2.0, IQR 1.0-3.0, p<0.001; Table 1, Fig 2b). Only 110 (30.1%) cases underwent definitive pleural treatment, including talc slurry (n=47, 12.9%) or poudrage (n=43, 11.8%) pleurodesis, and IPC placement (n=20, 5.5%). Talc pleurodesis failure rate was slightly higher in the group of patients with NEL, but without statistically significant difference between the groups (p=0.291). IPC was more frequently used in patients with NEL (n=14, 20.6% vs n=6, 2%; p=<0.001), and also in 6 cases following pleurodesis failure. Procedure-related complications are presented in Fig 1d.
OS was 3.0 (95%CI, 2.3-3.7) months, which was not significant influenced by lung entrapment (Table 1, Fig 2c). Nevertheless, patients with expandable lung had significantly shorter hospitalization days than patients with lung entrapment (median 21.0, IQR 11.0-36.0 vs 32.5, IQR 15.5-46.3 days, p=0.007, Fig 2d).
DiscussionThis study reports a prevalence of nearly 19% of NEL in a large cohort of cancer patients with pathologically confirmed MPE. Only a few studies have previously described the frequency of malignant lung entrapment, with varying rates according to the diagnostic criteria and histological subtypes. In a randomized trial (RCT) of patients with mixed MPE, NEL (defined as <75% pleural apposition) was found in 41 out of 923 patients (4.4%) at screening, and a further 32 of 250 patients (12.5%) were found to have NEL after a 10-day run-in period.15 Another RCT applied the criterion of <90% lung re-expansion to define lung entrapment, and reported a prevalence of nearly 30%.16 Given the inconsistency of NEL definition across studies, we decided to apply a more clinically oriented criterion, considering the recommendations to attempt a pleurodesis whenever ≥50% of visceral and parietal pleura are apposed.14 As such, NEL cases with <50% pleural apposition are, in fact, those that will have a more complicated management of MPE, given the reduced chemical pleurodesis efficacy.6 The treatment decision of MPE in these cases should be based on symptoms, patient's performance status and preferences, along with disease prognosis.7,17 While serial thoracocenteses may be appropriate for slowly reaccumulating pleural effusions and/or in patients with short survival expectancy, IPCs are generally accepted as the frontline approach to most cases of lung entrapment, enabling alleviation of symptoms at the ambulatory basis, without the need of repetitive invasive procedures. Nevertheless, aggressive fluid removal during drainage can induce chest discomfort due to tension on the NEL by negative intrathoracic pressure.18 Although some studies have reported good outcomes with surgical decortication,5 its definitive role in NEL is still uncertain. Results from MesoTRAP, a RCT comparing video-assisted thoracoscopic partial pleurectomy with IPC in patients with trapped lung due to malignant pleural mesothelioma19 may shed some light on this topic.
Considering the difficulties that lung entrapment poses on the symptoms palliation of cancer patients, and the high recurrence rate of MPE, it has been proposed that an early intervention may be beneficial in improving control of the pleural effusion, including a possible reduction of NEL risk.6 Although we did not assess symptoms control or quality of life, we demonstrated that patients with lung entrapment had more hospitalization days and underwent thoracocenteses more frequently than patients with expandable lungs. Considering the dismal prognosis associated with MPE, arguably these findings are inconsistent with the palliative treatment goals. For that reason, it may be useful to determine which patients are more prone to have NEL, in order to better select patients for a more aggressive approach of the MPE, namely early pleurodesis. Although late diagnosis of MPE is often mentioned as a risk factor for lung entrapment,6 we found no statistically significant differences between the median time from primary malignancy diagnosis or from symptoms onset to MPE diagnosis. In fact, when specifically considering lung cancer, the most represented malignancy, MPE was generally diagnosed at the same week as the primary cancer. Similar results were found by Porcel et al.,20 showing that patients with lung cancer are more likely to have pleural effusion at cancer diagnosis, whereas MPE is a late complication in other types of cancer.
In the present study, we confirmed the dismal prognosis associated with MPE,4,7 with a median OS of 3 months, but failed to show that NEL is associated with shorter survival.21-23 Although the presence of a pleural effusion reflects more advanced disease, most cases of lung entrapment could not be explained by diffuse or bulky metastatic spread along the pleural surface. Instead, we hypothesized that a thickened visceral pleura peel could be caused by local factors of the effusion, as explained below.
Most references in the literature point to the elastance of the pleural space as predictor for lung entrapment.24,25 These studies are usually small and do not investigate the impact of other clinical and pleural effusion features on the development of NEL. According to our analysis, loculated pleural effusion, complete white-out of a hemithorax, lung cancer and higher effusion-serum LDH ratio were all independent predictors for the occurrence of lung entrapment in patients with MPE. Septated or loculated pleural effusion are an obvious signs of NEL, as they limit the complete drainage of the pleural space.5 Likewise, complete hemithorax opacification usually result from a combination of pleural effusion, caused by direct tumor infiltration or metastatic invasion of the visceral pleura, and endobronchial obstruction causing distal lung collapse or atelectasis, which are both hallmarks of the NEL. Malignant airway obstruction may not be reversible, even when rigid bronchoscopy is available. We recently published our experience, showing that in patients with endobronchial obstruction causing lung atelectasis that were initially considered candidates for bronchoscopic intervention (selected based on clinical, endoscopic and radiological pre-assessment), the procedure was unsuccessful in 22% of cases.26 Technical failure was more often seen when distal airway patency was absent on thoracic CT, as is often the case in complete lung white-out. While loculations may be managed with intrapleural fibrinolytics administration,5 hemithorax opacification is hardly solved with a chest tube, and measures should be taken, when possible, to prevent the progression to this stage. Hence, we propose early intervention in MPE of lung cancer patients and/or with high effusion-serum LDH ratio on the first diagnostic thoracocentesis. Although increased pleural fluid protein levels were associated with lung entrapment, only high effusion-serum LDH ratio kept a significant association after multivariate analysis. Importantly, the finding of elevated LDH in the pleural fluid is an important indicator for the presence of active pleural disease, while protein concentration is more dependent on microvascular permeability.27 Lower glucose level was found to be a lung entrapment predictor in a previous study,24 but this association was not retrieved from our analysis.
Current guidelines5,6 recommend placement of IPCs in patients with symptomatic MPE with NEL, which in some cases could lead to pleural apposition overtime and enable pleurodesis. In the present cohort of MPE patients diagnosed between 2012-2018, serial thoracocenteses were the usual approach, and “definitive” pleural procedure, such as IPCs, were less commonly applied, even in cases of NEL. One large retrospective study of 23,431 patients with MPE demonstrated that only 24% underwent a definitive pleural procedure, talc pleurodesis or IPC placement, as opposed to repeat thoracentesis, after rapid fluid reaccumulation.28 Patients undergoing definitive pleural approach experienced fewer additional pleural procedures, including those performed in the emergency department, and fewer complications than cases managed with serial thoracocenteses, underlining the importance of definitive pleural intervention at the appropriate time. We admit that the issuance of guidelines may have an impact on the local habits regarding MPE management in the following years, and more cases with NEL will have an IPC. In our opinion, therapeutic drainage through thoracocentesis serves essentially 3 purposes: to confirm symptomatic improvement after fluid removal (thus, determining benefit at definitive pleural treatment), to identify NEL (to prevent futile attempts at pleurodesis), and to palliate symptoms in patients with poor performance status, with an estimated survival of less than one month.
Some limitations can be identified in our study. The present analysis is based on a single center, where local MPE management practices might influence the results presented. Moreover, its retrospective nature has the potential to introduce some information bias, and we lack data concerning the evolution of symptoms over time, which would be important to assess patients' quality of life and its relationship with the management of MPE. Despite that, we provide one of the largest cohort studies of patients with MPE and NEL in the recent literature. Prospective studies with patients with malignant lung entrapment should seek validation of our results and assess how the delay to MPE definitive treatment may compromise lung expandability and the patient's quality of life.
ConclusionsThis study gives an overview of our local MPE epidemiology and management options, and demonstrates that NEL is a relatively common phenomenon in patients with lung cancer and/or with higher effusion-serum LDH ratio. Moreover, the presence of loculations and complete hemithorax opacification are independent predictors for lung entrapment. Late diagnosis of MPE was not associated with a higher risk of NEL in our cohort, and no statistically significant difference regarding pleurodesis success or OS was found between NEL and expandable lung group.