Substance use disorder (SUD) causes conditions such as cognitive and behavioral disorders, anxiety, depression, and social isolation it also causes acute airway inflammation by affecting airway bronchial dynamics. The current study aimed to investigate the lung function, respiratory muscle strength, and exercise capacity in patients with SUD.
MethodsOne hundred-eighty three patients with SUD, a total of 119 healthy controls, 54 of whom were cigarette smokers and 65 of whom were non-smokers were included in the study. Spirometric tests, respiratory muscle strength (MIP and MEP), and the 6-Minute Walk Test (6-MWT) were assessed. The III National Health and Nutrition Examination Survey were used to evaluate respiratory symptoms in patients with SUD and cigarette smokers.
Results86.3% of the SUD patients included in the study were using heroin, 9.2% were cannabis, and 5.5% were spice. The most common symptom in both SUD patients and cigarette smokers was shortness of breath, wheezing, and sputum production. After post-hoc tests, the FVC (p = 0.002), FVC (%predicted) (p < 0.0001), FEV1 (p = 0.002), FEV1 (%predicted) (p < 0.0001), FEV1/FVC (%) (p < 0.0001), PEF (p < 0.0001) and FEF%25-75 (p < 0.0001) lung function parameters were significantly lower in SUD patients than non-smokers. In addition, it was found that MIP (p < 0.0001), MIP (%predicted) (p < 0.0001), MEP (p < 0.0001), and MEP (%predicted) (p < 0.0001) values of SUD patients were significantly lower than non-smokers.
ConclusionThe study findings indicate that substance use has an effect on lung functions and the most commonly reported symptoms are shortness of breath, wheezing, and sputum production. In addition, respiratory muscle strength and exercise capacity were decreased in SUD patients compared to non-smokers.
In recent years, substance abuse has become a serious and growing problem worldwide and has threatened economic, social, legal, and health systems as an essential health problem and social problem in developed and developing countries.1 It is estimated that one in four people in developed countries uses illicit drugs at some time in their life while one in six to seven people are at the risk of substance use disorder (SUD) in developing countries.2,3 The number of studies investigating the adverse effects of the substances in health is gradually increasing.4-6 Besides, SUD causes conditions such as cognitive and behavioral disorders, anxiety, depression, and social isolation it also causes acute airway inflammation by affecting airway bronchial dynamics and by preparing the ground for important histological changes in the airway mucosa.7-9
The current studies, investigating the acute and chronic effects of SUD on the respiratory system, showed that the acute effect of substances on the respiratory system is mostly on bronchial dynamics.10,11 Moreover, it has been reported that short-term substance use causes decreased airway resistance due to bronchodilation.12,13 Long-term substance use might result in symptoms such as cough and abnormal sputum production, causing obstruction, hyperinflation, and changes in respiratory functions in the airways.9,11,14,15 Recent studies have suggested that substance use for a long time may lead to chronic bronchitis by causing airway inflammation and infection. Also, individuals who use cannabis and cocaine for a long time become susceptible to bacterial and viral infections since these substances cause tracheobronchial mucosal damage in the airway.15 Moreover, substance use has been reported to cause respiratory symptoms such as wheezing, cough, shortness of exercise, increased sputum production.14,16,17 Substance use also affects pulmonary functions and leads to obstruction in the airways during long-term use.18-22
Many countries have national data showing the effect of substance use on respiratory functions.11,14,19-23 In recent years, substance use has been increasing among young people in Turkey. 24,25 To date, there are very limited studies on substance use in Turkey. Those studies reported data on substance use disorder prevalence, risk factors, sociodemographic determinants, and cognitive status.26-29 Studies on respiratory parameters in these individuals seem to be quite limited, and the need for such a study gains importance when the lack of research in this area is taken into account. However, accurate data to show the current situation in terms of the effects of substance use on the respiratory system is not available in Turkey. To the best of our knowledge, this is the first study to evaluate the respiratory muscle strength in patients with SUD. We therefore aimed to conduct a field study to obtain accurate data regarding lung functions, respiratory muscle strength, and exercise capacity in patients with SUD. We hypothesized that lung functions, respiratory muscle strength, and exercise capacity will be more affected in patients with SUD than in cigarette smokers and non-smokers.
MethodsA prospective, cross-sectional observational study was conducted in Bakirkoy Prof. Dr. Mazhar Osman Research and Training Hospital for Psychiatry, Neurology, and Neurosurgery, Research, Treatment, and Training Center for Alcohol and Substance Dependence from June 2018 to June 2019. Ethical approval was obtained from the Human Research Ethics Committee of Bakirkoy Dr. Sadi Konuk Training and Research Hospital (Approval number: 2018/42) and conducted in conformity with the Declaration of Helsinki. Verbal and written explanations about the study were provided to all the participants and written informed consents were obtained.
ParticipantsThe study sample consisted of 183 patients with SUD, a total of 119 healthy controls, 54 of whom were cigarette smokers and 65 of whom were non-smokers. Age-matched healthy controls consisted of hospital staff who met the eligibility criteria from the hospital staff list. Participants who were volunteers and met the inclusion criteria were enrolled in the study. The eligibility criteria for SUD participants were as follows; (1) age over 18 years old; (2) meeting current Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-V) criteria for SUD30; (3) using substances for over a year; (4) able to follow simple instructions; and (5) no pathology in visual ability and hearing. The eligibility criteria for cigarette smoker participants were as follows; (1) age over 18 years old; (2) smoking for over a year (active smokers); (3) able to follow simple instructions; and (4) no pathology in visual ability and hearing. The eligibility criteria for non-cigarette smoker participants were as follows; (1) age over 18 years old; (2) never smoked tobacco products (never smokers); (3) able to follow simple instructions; and (4) no pathology in visual ability and hearing. For all participants, the exclusion criteria were as follows: (1) current psychotic symptoms, (2) no physical disabilities (e.g., lower limb fractures, contractures) or medical problems (e.g., hypertension, heart attack, diabetes), or (3) respiratory system problems such as bronchiectasis, asthma, and tuberculosis, infectious health problem (e.g., HIV, hepatitis B).
MeasurementsThe socio-demographic information of the participants was recorded. In addition, pulmonary function tests, respiratory muscle strength, and 6-Minute Walk Test (6-MWT) were evaluated in the study. Furthermore, the dyspnea severity was assessed using the Modified Medical Research Council Dyspnoea (mMRC), and nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND) among current cigarette smokers. The III National Health and Nutrition Examination Survey (NHANES III) was used to evaluate respiratory symptoms in patients with SUD and cigarette smokers.
DyspneaAll participants' perceptions of dyspnea in daily living were evaluated using the modified Medical Research Council (mMRC) scale, which consists of five statements that describe almost the entire range of dyspnea from none (Grade 0) to almost complete incapacity (Grade 4).31
Nicotine dependenceThe FTND, which is a standard instrument for assessing the intensity of physical addiction to nicotine, is used to measure the nicotine dependence related to cigarette smoking. It contains six items that evaluate the quantity of cigarette consumption, the compulsion to use, and dependence. The items are summed to yield a total score of 0-10 with a higher score indicating higher nicotine dependence.32,33
Lung function testsLung function was measured using portable spirometry (Spirobank II; MIR, Rome, Italy). Measurements were performed according to the criteria of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) guidelines.34 Instructions and demonstrations were given to the participants before the spirometry measurements were taken. After three acceptable maneuvers, the highest values were selected for analysis.35 Measurements were specified as percentages of the predicted values.
Respiratory muscle strengthThe respiratory muscle strength was evaluated by the maximum inspiratory pressure (MIP) and maximum expiratory pressure (MEP) according to ATS/ERS criteria using a portable MicroRPM device (Micro Medical, Basingstoke, UK). The highest value from five acceptable and reproducible attempts was recorded (i.e., a difference of ≤ 10% among values) and is expressed as an absolute value (cmH2O).36 A percentage of the predicted values of MIP and MEP was specified as described by Black and Hyatt.37
Exercise capacityThe 6-MWT, which is a reliable and valid test for evaluating exercise capacity, was performed according to the guideline of the ERS/ATS.38 Systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), perceived dyspnoea, and fatigue as measured using modified Borg scale (mBorg) were assessed before and after the 6-MWTs. The distance in meters covered over the 6 minutes was recorded.39 Also, the percentage of the predicted values was specified as described by Enright and Sherril.40
Statistical analysisStatistical Package for Social Science (SPSS) version 21.0 for Windows software (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. Descriptive statistics, including frequency, the percentage for nominal variables, and mean and standard deviation for continuous variables were calculated. The Kolmogorov-Smirnov test was used to test the normality of data distribution. Demographic data were compared among the three groups by one-way analysis of variance (one-way ANOVA) for continuous variables and Chi-squared test for categorical variables. Dependent variables (lung function test, respiratory muscle strength, and the 6-MWT) were compared among the three groups by multivariate analysis of variance (MANOVA). In addition, covariance analysis (MANCOVA) was used in statistical analysis. The sex, age, weight, height, BMI, number of cigarettes smoked per day, duration of smoking, and score of the FTND were used as covariates, as they have an impact on the dependent variables. Once differences among the means were determined, the least significant difference (LSD) post hoc test was used. The significance level was set at p < 0.05.
The estimated sample size was derived from the online Raosoft sample size calculator (RaoSoft, Inc., Seattle, WA; http://www.raosoft.com/samplesize.html). Raosof online calculator is designed specifically for population surveys to calculate the sample size and determine how many responses are needed, to meet the desired confidence level with the margin of error (usually 5%). Therefore, it is highly recommended to be used for such a study with the consideration of the population size.41 The total population of heroin users in Istanbul is approximately 46.500.28 Therefore, in order to achieve a confidence level of 90%, a response rate of 50%, and a 5% margin of error, a minimum sample size of 271 was required. However, from a total of only 217 patients with SUD screened for eligibility criteria, only 183 patients with SUD were enrolled in the study. A sample of 183 patients with SUD would provide a confidence level of 90% and a 6.08% margin of error which may affect the power of the study.
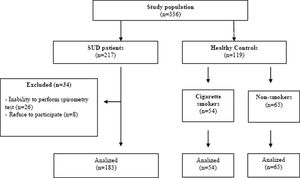
ResultsA total of 217 patients with SUD who met the inclusion criteria were enrolled in the study. A total of 34 patients with SUD patients were excluded from the study because 26 of the participants could not comply with the spirometry test and 8 people refused to participate in the study voluntarily. As a result, a total of 302 volunteers, including 183 patients with SUD, a total of 119 healthy controls, 54 of whom were cigarette smokers and 65 of whom were non-smokers in the same age group were included in the study (Fig. 1).
Demographic and clinical characteristicsA comparison of the demographic and clinical characteristics of the participants is given in Table 1. The weight and BMI of SUD patients were significantly less than cigarette smokers and non-smokers (p = 0.013 and p = 0.042; p = 0.018 and p = 0.048, respectively). The mMRC score of SUD patients was significantly higher than cigarette smokers and non-smokers (p < 0.0001 and p < 0.0001, respectively). In addition to cigarette smoking, 86.3% of SUD patients were using heroin, 9.2% cannabis, and 5.5% spices. However, it is seen that some of the SUD patients in the study were polysubstance users, 37.1% of the patients used more than one type of substance, and 16.9% used at least 3 types of substances.
Comparison of the clinical and socio-demographic characteristics of the participants.
| SUDs (n = 183) | Cigarette Smokers (n = 54) | Non-smokers (n = 65) | p*,# | |
|---|---|---|---|---|
| Gender -Female/Male | 15(8.2)/168(91.2) | 5(9.3)/49(90.7) | 6(9.2)/59(90.8) | 0.951 |
| Age (year) | 29.77±8,.1 | 29.61±9.99 | 28.71±9.49 | 0.692 |
| Height (cm) | 173.72±7.13 | 175.09±7.58 | 175.46±7.41 | 0.182 |
| Weight (kg) | 68.60±10.66 | 73.59±11.37 | 73.08±12.51 | 0.002a |
| BMI (kg/cm2) | 22.71±3.14 | 23.94±2.94 | 23.78±4.39 | 0.011b |
| Education period (year) | 8.43±2.70 | 13.65±2.96 | 14.45±2.56 | <0.0001c |
| Age of starting substance use (year) | 17.99±5.37 | - | - | Na |
| Duration of substance use (year) | 11.28±6.56 | - | - | Na |
| Distribution of the substances used | ||||
| Heroin | 158 (86.3) | - | - | Na |
| Cannabis | 17 (9.3) | - | - | Na |
| Spice | 8 (4.4) | - | - | Na |
| Age of starting smoking cigarette (year) | 14.28±3.52 | 19.30±2.86 | - | <0.001 |
| Duration of cigarette smoking (year) | 15.24±7.41 | 10.33±8.76 | - | <0.001 |
| Cigarette smoking (packs/year) | 20.7±15.8 | 10.2±12.6 | - | <0.001 |
| FTND score | 5.81±1.92 | 2.90±1.52 | - | <0.001 |
| mMRC | 1.54±0.78 | 0.55±0.76 | 0.00±0.00 | <0.0001d |
Data expressed as number (percentage) or mean ±SD for qualitative variables.
Abbreviations: FTND: Fagerström Test for Nicotine Dependence, mMRC: Modified Medical Research Council, na: not available
The weight of SUD patients was statistically significantly lower than cigarette smokers and non-smokers (p = 0.013 and p = 0.018, respectively).
The SUD patients BMI was significantly lower than those cigarette smokers and no-smokers (p = 0.042 and p = 0.048, respectively).
Self-reported respiratory symptoms in SUD patients and cigarette smokers are presented in Table 2. It was observed that the most common symptoms in both SUD patients and cigarette smokers were shortness of breath when walking in a hurry or climbing slightly uphill, wheezing or whistling sound, and sputum. Except for chronic bronchitis and pneumonia, other respiratory-related symptoms in SUD patients were found to be statistically more common than cigarette smokers (p < 0.05).
The NHANES III respiratory symptoms in SUD patients, cigarette smokers, and non-smokers.
Data expressed as number (percentage).
Abbreviations: NHANES: National Health Nutrition Examination Survey.
There was a statistically significant difference between the mean scores of lung function test parameters between the groups [Wilks’ Lambda (Ʌ)=0.80, F=4.88; p < 0.0001, Ƞ2=0.10]. After post-hoc tests, the FVC (p = 0.002), FVC (%predicted) (p < 0.0001), FEV1 (p = 0.002), FEV1 (%predicted) (p < 0.0001), FEV1/FVC (%) (p < 0.0001), PEF (p < 0.0001) and FEF%25-75 (p < 0.0001) parameters were statistically significantly lower in SUD patients than non-smokers. In addition, it was found that FVC (%predicted) (p = 0.016), FEV1 (%predicted) (p < 0.003), FEV1/FVC (%) (p = 0.013), PEF (p < 0.001), and FEF25-75% (p = 0.008) values were significantly lower in cigarette smokers compared to non-smokers (Table 3). Gender [F=5.66, p < 0.0001, Ƞ2=0.12] and duration of cigarette smoking [F= 6.70, p < 0.0001, Ƞ2=0.14] were found as confounding factors for lung function test parameters. Similarly, there was a statistically significant difference between the groups in respiratory muscle strength values [Wilks’ Lambda (Ʌ)=0.91, F=5.75; p < 0.0001, Ƞ2=0.04]. After post-hoc tests, MIP (p < 0.0001), MIP (%predicted) (p < 0.0001), MEP (p < 0.0001), and MEP (%predicted) (p < 0.0001) values of SUD patients were significantly lower than non-smokers. In addition, it was found that MIP (%predicted) (p < 0.024) and MEP (%predicted) (p < 0.027) values were significantly lower in cigarette smokers compared to non-smokers (Table 3). Duration of cigarette smoking was found to be a confounding factor for respiratory muscle strength parameters [F=6.82, p < 0.0001, Ƞ2 = 0.04] However, other confounding factors had no effects on both lung functions and respiratory muscle strength.
Comparisons of the spirometry parameters and respiratory muscle strength values of the participants.
| SUDs (n = 183) | Cigarette Smokers(n = 54) | Non-smokers (n = 65) | F | p# | Post-hoc analysis p- values | |
|---|---|---|---|---|---|---|
| FVC (L) | 4.74±0.87 | 4.86±1.04 | 5.19±0.89 | 4.43 | 0.003 | SUD-CS: 0.576SUD-NS: 0.002CS-NS: 0.059 |
| FVC, % predicted | 89.50±17.02 | 94.21±21.51 | 102.40±18.34 | 11.46 | <0.0001 | SUD-CS: 0.105SUD-NS: <0.0001CS-NS: 0.016 |
| FEV1 (L) | 3.93±0.78 | 4.01±0.91 | 4.32±0.67 | 5.28 | 0.003 | SUD-CS: 0.520SUD-NS: 0.002CS-NS: 0.061 |
| FEV1, % predicted | 89.50±17.02 | 89.50±17.02 | 89.50±17.02 | 9.27 | <0.0001 | SUD-CS: 0.598SUD-NS: <0.0001CS-NS: 0.003 |
| FEV1/FEVC (%) | 81.22±8.46 | 83.58±7.85 | 87.91±7.50 | 21.25 | <0.0001 | SUD-CS: 0.127SUD-NS: <0.0001CS-NS: 0.013 |
| PEF (L/s) | 7.34±2.09 | 7.54±2.02 | 8.93±1.90 | 3.75 | <0.0001 | SUD-CS: 0.126SUD-NS: <0.0001CS-NS: <0.001 |
| FEF%25-75 (L/s) | 4.17±1.32 | 4.33±1.23 | 5.06±1.31 | 11.10 | <0.0001 | SUD-CS: 0.373SUD-NS: <0.0001CS-NS: 0.008 |
| FEV3 (L) | 4.78±0.84 | 4.66±1.12 | 4.90±0.80 | 0.62 | 0.351 | |
| FEV6 (L) | 4.75±0.85 | 4.72±1.13 | 5.10±0.89 | 2.44 | 0.022 | |
| MIP (cmH2O) | 89.51±26.09 | 94.88±24.87 | 105.61±30.10 | 4.20 | <0.0001 | SUD-CS: 0.072SUD-NS: <0.0001CS-NS: 0.672 |
| MIP, %predicted | 70.39±21.33 | 72.33±18.32 | 83.44±26.97 | 7.70 | <0.0001 | SUD-CS: 0.393SUD-NS: <0.0001CS-NS: 0.024 |
| MEP (cmH2O) | 110.23±36.96 | 121.72±28.42 | 136.24±38.28 | 2.72 | <0.0001 | SUD-CS: 0.157SUD-NS: <0.0001CS-NS: 0.674 |
| MEP, %predicted | 47.08±15.60 | 50.82±12.96 | 57.26±11.56 | 10.68 | <0.0001 | SUD-CS: 0.132SUD-NS: <0.0001CS-NS: 0.027 |
Data expressed as mean ±SD for qualitative variables.
Abbreviations: FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in the First Second; PEF: Peak Expiratory Flow; FEF25-75%: Forced Mid-Expiratory Flow; FEV3: Forced Expiratory Volume in 3 Seconds; FEV6: Forced Expiratory Volume in 6 Seconds; MIP: Maximum Inspiratory Pressure; MEP: Maximum Expiratory Pressure; SUD: Substance Use Disorders; CS: Cigarette Smokers; NS: Non-Smokers.
A statistically significant difference was found between the groups in terms of 6-MWT [Wilks’ Lambda (Ʌ)=0.54, F=17.08; p < 0.0001, Ƞ2=0.25]. After post-hoc tests, a statistically significant difference was found between the SUD patients and non-smokers in terms of 6-MWD (p < 0.0001), 6-MWT (%predicted), (p = 0.006), ΔHR (p < 0.0001), ΔSBP (p = 0.005), ΔDyspnea (Borg) (p < 0.0001) and ΔPerceived Exertion (Borg) (p < 0.0001). It was observed that ΔHR (p < 0.0001) and ΔSBP (p = 0.005) were significantly higher in SUD patients compared to cigarette smokers. In addition, it was found that ΔDyspnea (Borg) (p < 0.0001) and ΔPerceived Exertion (Borg) (p < 0.0001) were significantly higher in the cigarette smokers compared to the non-smokers (Table 4). Duration of cigarette smoking was found to be a confounding factor for exercise capacity parameters [F=2.29, p = 0.035, Ƞ2=0.04]. However, other confounding factors had no effects on exercise capacity parameters.
Comparison of the functional exercise capacities of the participants.
| SUDs(n = 183) | Cigarette Smokers (n = 54) | Non-smokers (n = 65) | F | p# | Post-hoc analysisp- values | |
|---|---|---|---|---|---|---|
| 6-MWT (m) | 512.17±80.60 | 535.46±76.88 | 577.45±93.33 | 14.77 | <0.0001 | SUD-CS:0.091SUD-NS: <0.0001CS-NS: 0.059 |
| 6-MWT, %predicted | 69.87±10.49 | 71.88±11.97 | 78.56±13.72 | 3.98 | 0.02 | SUD-CS: 0.962SUD-NS: 0.006CS-NS: 0.051 |
| ΔHR | +25.86±21.50 | +11.85±7.80 | +6.95±8.82 | 33.63 | <0.0001 | SUD-CS: <0.0001SUD-NS: <0.0001CS-NS: 0.063 |
| ΔSBP | +13.12±13.79 | +5.85±9.72 | +7.55±8.49 | 10.23 | <0.0001 | SUD-CS: 0.005SUD-NS: 0.005CS-NS: 0.259 |
| ΔDBP | +6.07±12.74 | +4.40±8.09 | +3.52±6.01 | 1.63 | 0.198 | |
| ΔDyspnea (Borg) | +1.30±0.88 | +1.18±0.95 | +0.16±0.45 | 46.47 | <0.0001 | SUD-CS: 0.934SUD-NS: <0.0001CS-NS: <0.0001 |
| Δ Perceived Exertion (Borg) | +1.44±0.75 | +1.37±1.26 | +0.33±0.71 | 40.94 | <0.0001 | SUD-CS: 0.896SUD-NS: <0.0001CS-NS: <0.0001 |
Data expressed as mean ±SD for qualitative variables.
Abbreviations: Δ: Change before and after 6-MWT; HR: Heart Rate; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; SUD: Substance Use Disorders;
CS: Cigarette Smokers; NS: Non-Smokers.
In this cross-sectional study, we observed that SUD patients started smoking earlier, smoked longer periods, consumed more cigarettes during the day, and had higher levels of nicotine addiction level than cigarette smokers. It was found that the most common symptoms in both SUD patients and cigarette smokers were shortness of breath, wheezing, and sputum production. In addition, SUD patients' lung function test parameters were significantly decreased compared to non-smokers, gender and smoking duration were confounding factors for lung function test parameters. Also, SUD patients had significantly lower respiratory muscle strength and exercise capacity compared to non-smokers, and smoking duration was found to be a confounding factor.
In this study, 91.2% of SUD patients were male. Similarly, Demirci et al.25 reported in their study that 82.4% of the participants were male. Charitonidi et al.42 also reported that 50.1% of SUD patients had primary and secondary education. Büker et al.,43 in their study on adolescent SUD patients, reported that 90.3% of the cases did not attend school and the average education period of the participants was 7.8 years. Similarly, in our study, we found that the education period of the SUD patients was 8.4 years. According to these findings, we can say that males constitute a significant portion of individuals using substances. The addiction to substance use may cause disruptions in education as well as negatively affect their health.
In the literature, the age of initiation of substance was reported as 14 years by Buchowski et al.44 16 years by Flemmen et al.,45,46 and 13.8 years in the study of Demirci et al.25 In another study, Büker et al.43 reported the age of initiation substance use 14.6 years and the duration of substance use was 3.7 years. In our study, it was observed that the mean age of initiation of substance was 17.9 years, and the mean duration of use was 11.2 years. Flemmen et al.45,46 reported in their studies that the preferred substances for use by the SUD patients were heroin, cannabis, benzodiazopem, and ecstasy. Roessler 47 determined that the substances used by the SUD patients were heroin, ecstasy, cannabis, cocaine, benzodiazopem, while Demirci et al.25 reported that cannabis, alcohol, spice, ecstasy, solvent/inhalants, benzodiazopem, and other substances. Burhan et al.48 reported that 99% of individuals participating in their studies used cigarettes, 98% used heroin, 83% crack cocaine, and 83% cannabis. In our study, 86.3% of the participants were using heroin, 9.2% cannabis and 5.5% spice. In addition, 37.1% of the participants were found to use more than one type of substance (cocaine, ecstasy, etc.). As seen in the literature, SUD patients start using these substances from an early age and use more than one type of substance. In addition to the use of cigarettes, none of the participants in the study used a single type of substance, but users preferred one of them (heroin, cannabis and spices, etc.) more intensely than the others. In this case, it is seen that the SUD patients in the study were polysubstance users. Thus, it is impossible to determine from which substances the observed effects are caused. This condition seriously threatens both the respiratory and physical health of individuals.
Most, although not all, researchers have described increased reporting of respiratory symptoms among SUD patients similar to our findings.11,23,49 Macleod et al.23 reported that in the cannabis users, the prevalence of chronic bronchitis is 14.7%, sputum production is 38.5%, shortness of breath is 47.5%, wheezing is 66.3%, and pneumonia is 3.6%. Also, Aldington et al.11 reported that 28.6% of cannabis users had a cough, 34.1% wheezing, and 30.8% chronic bronchitis. In the current study, 74.9% of the SUD patients had shortness of breath, 66.1% had wheezing, and 63.9% had sputum production. Similarly, 46.93% of cigarette smokers had shortness of breath, 48.1% had wheezing, and 38.9% had sputum production. Boto de los Bueis et al50 found a 41.9% prevalence of wheezing, a 44.4% prevalence of bronchial hyperreactivity, and a 22.03% prevalence of asthma among subjects who inhaled a mixture of heroin and cocaine vaporized on aluminum foil. Besides, Buster et al.49 reported that 45% of individuals using heroin had mMRC dyspnea scale scores 0, 15% had 1, 18% had 2, and 22% had 3 and above. In our study, the distribution of mMRC dyspnea scale scores in SUD patients was 0 in 8.2%, 1 in 39.3%, 2 in 42.1%, and 3 and above in 10.4%. We think that the difference between the results of our study and the results of this study may be due to the presence of cannabis and spice users together with heroin in our study. Inhaling heroin and other substances involves repeated exposure to irritants rather than a single exposure to high concentrations of vapor. The physiopathogenic mechanism underlying the reactive airway is thought to cause an abnormal re-epithelialization and re-innervation of the bronchial mucosa after epithelial damage caused by initial exposure to the toxic substance. This can result in hypersensitivity of subepithelial receptors and, consequently, to maintained airway hyperresponsiveness.50 Based on these possible mechanisms, evidence from this study suggests that SUD patients report more respiratory symptoms than smokers.
In recent years, substance use has been increasing in Turkey and in the rest of the world. Accordingly, the number of studies investigating the harmful effects of substance use on health is increasing. Substance use has been reported to affect lung functions, decrease FVC and FEV1, and predispose to airway obstruction in long-term use.18,20-22,51,52 Macleod et al.23 stated in their study that FVC was notably higher among cannabis users. In another study, Aldington et al.11 found that both cannabis and tobacco use were associated with a decrease in the FEV1/FVC, cannabis use did not affect FEV1, and tobacco use caused a decrease in FEV1. According to the authors, smoking cannabis was associated with a dose-related impairment of large airways function resulting in airflow obstruction and hyperinflation Similarly, Taylor et al.,18 reported that there was a linear relationship between cannabis use and FEV1/VC, and increased cannabis use over time was associated with a decrease in FEV1/VC over time. It has been suggested that age, smoking, and weight are important determinants of FEV1/VC, with marijuana use and daily smoking additionally affecting FEV1/VC. Hancox et al.,21 suggested that cannabis was associated with evidence of hyperinflation and increased large airway resistance, with little evidence of airflow obstruction or impairment of gas transfer, whereas tobacco was associated with airflow obstruction, gas trapping, and lower transfer factors. According to the authors, smoking cannabis and tobacco have different physiological consequences for the lungs. Samoedro et al.,22 reported that there was a weak correlation between declined FEV1/FVC with length of time of smoking, the amount of cigarette consumption per day, time of cannabis inhalation, time of methamphetamine inhalation, and time of heroin injection. Buster et al.49 reported a difference in FEV1 from predicted values, finding that heroin smokers had an FEV1 of 260 ml less than predicted FEV1. Nightingale et al.,53 found that lung function measured by FEV1 declined by 90 ml annually, which was both statistically and clinically significant. Walker et al.54 found heroin smokers developed early-onset emphysema, with a mean age of diagnosis being 41 years, suggesting likely early progression of disease compared with non heroin smokers. Burhan et al48 reported that just under one-half of 753 heroin smoker people had fixed airflow obstruction with an FEV1/FVC <0.7. In our study, it was observed that the FVC, FVC (%predicted), FEV1, FEV1 (%predicted), FEV1/FVC, PEF, and FEF25-75% were decreased in SUD patients compared to non-smokers. In addition, cigarette smokers were found to be significantly lower in FVC (%predicted), FEV1 (%predicted), FEV1/FVC, PEF, and FEF25-75% compared to non-smokers. Sex and duration of smoking were found to be confounding factors for lung function test parameters in SUD patients. In addition to cigarette smoking, the possible effect of heroin on the respiratory system is an increase in histamine release that causes pulmonary vein constriction, an increase in pulmonary capillary permeability, leading to pulmonary edema, bronchospasm, and hypersensitivity pneumonia.55 Another possible mechanism is that it affects respiratory control centers, which can lead to fatal pulmonary depression.56 The rapid decline in lung functions and the increase in respiratory symptoms in this population suggest heroin smoking is a driver of the decline in lung function. Some of the discrepancies between the present findings and those of previous studies may be attributable to the relatively high levels of cumulative exposure seen in the present study population. In addition, in the current study, MIP, MIP (%predicted), MEP, and MEP (%predicted) values of the SUD patients significantly lower than the non-smokers. Although both MIP and MEP values were lower in SUD patients compared to cigarette smokers, this decrease was not significant. In addition, MIP (%predicted), and MEP (%predicted) were significantly lower in cigarette smokers compared to non-smokers. The smoking duration was found to be a confounding factor for respiratory muscle strength parameters. There is no study evaluating the respiratory muscle strength of SUD patients in the literature, and in this respect, our study is the first in this regard. The possible mechanism of decrease in respiratory muscle strength is the release of free radicals that occur in cigarette smoking and substance use into the vascular system, resulting in decreased blood flow and gas exchange to the respiratory muscle, which adversely affects respiratory muscle performance.
The number of studies investigating exercise capacity in SUD patients is limited in the literature. Patients with SUD are generally physically incompetent. This situation is associated with both unhealthy living habits and the effects of the substances used.57-59 Dolezal et al.56 reported that VO2max levels for men and women using methamphetamine were 30.6 and 23.2 mL/kg/min respectively. These values, based on the well-established reference values for age and sex, are classified as having poor cardiorespiratory fitness, with average rankings below 10% percentile for age and sex. Gimenez-Meseguer et al.,59 similar to our study, evaluated the exercise capacities of the participants with the 6-MWT. It was reported that 6-MWT results before treatment in the experimental group were 618.8 meters and 623.2 meters in the control group. In our study, the 6-MWT results of SUD patients were found to be 512.17 meters, this value was 535.46 meters for cigarette smokers and 577.45 meters for non-smokers. In addition, there were significant differences in 6-MWT, mean heart rate, mean systolic blood pressure, shortness of breath, and fatigue scores of SUD patients compared to non-smokers. In this population, this may be due to decreased lung function and respiratory muscle strength, resulting in decreased exercise capacity. As a result, many SUD patients show physical impairment and a low level of fitness compared to the general population due to the nature of the substances they use. Improved exercise capacity may be important for SUD patients for the prevention or mitigation of a wide range of physical comorbidities.
Strengths and limitationsThe main strengths of this study are that it is a general practice-based sample of non-smokers, cigarette smokers, and SUD patients. This study is also the first study in the literature to evaluate respiratory muscle strength in patients with SUD. The main limitations were that the study was cross-sectional and limited causality inference. Another limitation of the study is the inability to perform other laboratory and field measurements such as lower limb muscle strength, physical activity, cardiopulmonary exercise test to better define the limitations of SUD patients. In addition, we lacked data on the possible effects of substance use over the lifetime of the participants, individual differences in inhalation methods used by cigarette smokers only, and both cigarette and substance smokers.
Clinical implications of the study and future researchThis is the first study from Turkey providing data on the potential impact of SUD on lung functions, respiratory muscle strength, and exercise capacity. It is also the first study in the literature to provide results on respiratory muscle strength in SUD patients. The study findings indicate that there are some adverse respiratory effects from smoking substances and cigarettes. Limited data suggested that smoking both tobacco and substance may have additive adverse respiratory effects and long term substance use has been linked to an increase in respiratory symptoms.11,12,60 In the current study the most common respiratory symptoms in both SUD patients and cigarette smokers were shortness of breath, wheezing, and sputum production. The adverse effects of cigarette smoking on the lungs are well established. By contrast, the potential impact on lung health of substance use, with its wide range of toxins, is poorly understood. Smoking both cigarettes and substances may synergistically increase the risk of respiratory symptoms. Future studies with larger cohorts are needed, possibly in the context of a targeted public health intervention, to understand how best to avoid the personal and health costs associated with chronic respiratory disease. Moreover, studies should examine user characteristics associated with use trajectory groupings across primary drug types, and identifying factors associated with different lifetime drug use patterns will assist in the development of more targeted treatment services and policies. However, randomized controlled clinical trials will be needed to assess whether treatment services will be clinically and cost-effective in this population and how they will impact respiratory-related problems.
ConclusionThis study is the first study from Turkey providing data on the potential impact of SUD on the prevalence of respiratory symptoms, lung functions, and exercise capacity in a general practice population. The study findings indicate that substance use has an effect on lung functions and the most commonly reported symptoms are shortness of breath, wheezing, and sputum production. In addition, a decrease was observed in respiratory muscle strength and exercise capacity compared to non-smokers.
Ethical approvalAll procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. Moreover, informed consent was obtained from all individuals included in the study (Approval number: 2018/42).
FundingThis study was supported by the Turkish Thoracic Society (Project No: Y-110/2018); however, it did not influence the interpretation of the results and conclusions obtained in the present study.
The authors would like to thank Dr. Ishtiaq Ahmed PT for his editing support.
We are grateful to our patients who participated in the study.