Pulmonary rehabilitation is an evidence-based, multidisciplinary, comprehensive intervention for patients with chronic respiratory diseases who are symptomatic and whose daily living activities are often restricted.
Pulmonary rehabilitation programs are designed to improve the physical and emotional condition of people with chronic respiratory disease and to promote long-term adherence to health-enhancing behavior.
Exercise training is at the core of pulmonary rehabilitation (PR) programs. The benefits of exercise training include decreased dyspnea, improved health-related quality of life, fewer days of hospitalization, and decreased health-care utilization.
To gain PR benefits, patients should be able to complete an exercise training program, preferably with high intensity exercise, and it is likely that these benefits will translate into a change from a pattern of a sedentary lifestyle to a physically active lifestyle.
Chronic respiratory patients, namely COPD patients, have a low exercise tolerance due to multiple factors, such as dynamic hyperinflation and peripheral muscle dysfunction.
In this article, the authors describe a variety of modalities and strategies to overcome exercise limitations and improve the effects of exercise training.
A reabilitação pulmonar é uma intervenção abrangente, multidisciplinar e baseada em evidências, para doentes com doenças respiratórias crónicas que são sintomáticas e cujas actividades da vida diária são frequentemente limitadas.
Os programas de reabilitação pulmonar estão concebidos para melhorar a condição física e emocional de pessoas com doenças respiratórias crónicas e promover a adesão a longo prazo a comportamentos benéficos para a saúde.
O exercício físico está no cerne dos programas de reabilitação pulmonar (RP). Os benefícios do exercício físico incluem redução da dispneia, melhor qualidade de vida em termos de saúde, menos dias de hospitalização, e utilização reduzida dos cuidados de saúde.
Para obter os benefícios da RP, os doentes deverão ser capazes de completar um programa de exercício físico, de preferência com exercícios de alta intensidade, e é provável que esses benefícios se traduzam numa mudança de um padrão de estilo de vida sedentário para um estilo de vida fisicamente activo.
Doentes com doenças respiratórias crónicas, nomeadamente com DPOC, têm uma baixa tolerância ao exercício devido a uma diversidade de factores, como a hiperinsuflação dinâmica e a disfunção muscular periférica.
Neste artigo, os autores descrevem uma variedade de modalidades e estratégias para superar as limitações de exercício e melhorar os efeitos do treino físico.
Exercise training (ET) is a mandatory component of pulmonary rehabilitation (PR), it is at the core of PR programs and improves patients exercise tolerance and functional capacity, fatigue and dyspnea symptoms and health-related quality of life.1–4
Pulmonary rehabilitation also has benefits for health-related costs, reducing the number of exacerbations and hospitalizations, days in hospital and mortality in COPD patients.5
Although highly recommended by scientific societies, at present, less than 5% of eligible patients have access to pulmonary rehabilitation.6 There is a need to optimize availability, accessibility and quality of PR in Europe, and that includes Portugal.
According to GOLD, exercise training has benefits for all categories of COPD, but PR programs are mainly directed at the most symptomatic and severe patients: grades B, C and D.7
Exercise intensity is a key element in improving outcomes. Therefore, high intensity training is recommended for its physiological benefits.2
In this article, authors describe a variety of modalities and strategies to overcome exercise limitations and improve the effects of exercise training.
Exercise training componentsExercise training modalities include:
- 1.
Endurance training:
- (a)
Lower and upper limbs
- (b)
Continuous or interval training
- (c)
High and moderate intensity. Relevance of total dose (combining training intensity, training session duration and frequency)
- (a)
- 2.
Strength training: lower and upper extremities
- 3.
Neuromuscular electrical stimulation (NMES)
Limiting factors of exercise performance in COPD patients are multifactorial,8 but dynamic hyperinflation and peripheral muscle dysfunction are the most relevant ones, particularly in severe patients.
Strategies to enhance exercise toleranceMultiple strategies to improve tolerance to high intensity exercise training in severe patients have been presented in literature9:
- •
Pharmacological treatment optimization10,11
- •
Non-invasive ventilation (NIV)12,13
- •
Oxygen supplementation (O2)14–18
- •
Heliox19–21
- •
Aerobic training modalities: interval training,22–28 bicycle interval training,29 nonlinear exercise training30
- •
Localized muscle training: a few muscle groups are active at each moment31–32 bicycle single-leg (unilateral),32 progressive strength training (or isolated quadriceps training)33,34
- •
Neuromuscular electrical stimulation (NMES)35–37
- •
Eccentric exercise training (experimental)38,39
- •
Walking aids utilization40,41
In all above strategies, pharmacological treatment optimization with inhaled bronchodilators is highly recommended to improve symptoms and dynamic lung hyperinflation and to enhance exercise tolerance. It is essential to prescribe them appropriately, to stimulate patient compliance and verify inhalation technique.
Noninvasive ventilation, oxygen therapy and heliox supplements during exercise training are matters of debates. The real value of these exercise training adjuncts is not well established.
Noninvasive ventilation and heliox supplements have been the subject of pilot studies that have shown evidence of exercise tolerance improvement in patients who have severely limited ventilation, but their efficacy and applicability has not been established.
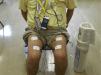
Use of supplemental oxygen in patients with exercise dessaturation (Fig. 1) is consensual in order to maintain an oxygen saturation of at least 90% during the training session (grade of recommendation 1C).2
Some studies have suggested the benefit of oxygen supplementation in exercise tolerance in non-hypoxemic patients,18 but the effects in improving activities of daily living, health status, morbidity or mortality are not yet documented. This therapy might be cost-effective in ventilatory limited patients where dyspnea due to dynamic hyperinflation prevents high intensity exercise training. Oxygen supplementation reduces dynamic hyperinflation through inhibition of carotid sinus stimulation, reducing ventilatory drive and respiratory rate. During exercise sessions it is also known to improve oxygen delivery to peripheral muscles, reducing muscle fatigue, which underpins its role as a training adjuvant and potentiating its effects.
Exercise training is of paramount value in improving exercise tolerance. It reduces ventilatory demands for sub maximal efforts due to a lower lactic acid production in muscle fibers. This will reduce respiratory rate, and hence the dynamic hyperinflation for the same exercise intensity.
Exercise intensity and session duration are determinants of the physiological response to training. However, continuous high intensity exercise is usually too hard for patients to achieve due to their ventilatory limitation, their muscle dysfunction with its premature lactic acidosis, their higher ventilatory demand and their dynamic hyperinflation.
Some exercise training modalities are also good strategies for improvingThis is the case with interval training, which produces a high intensity peripheral muscle demand and at the same time, a lower ventilatory demand due to late lactic acidosis. Interval training with repeated short high intensity training periods (30 s to 3 min), interspersed with rest or low intensity periods, is well suited for patients with dynamic hyperinflation, and it delays its onset. Interval training is well suited for patients with dynamic hyperinflation, and it delays its onset. The benefits are well documented, it not only improves training tolerance, but it also enhances muscle fiber capillarity, metabolism, typology and size.
Muscle training involving fewer muscle groups, namely quadriceps strength training, could be an alternative option, particularly in patients with a high risk of deconditioning (e.g. severe exacerbations with hospitalization).
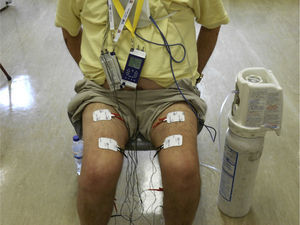
Neuromuscular electrical stimulation of ambulatory muscles (Fig. 2) might be an additional or alternative strategy in very disabled patients, such as patients with acute exacerbations or patients on an active list for lung transplant.
All the above strategies or training modalities could be either alternative or complementary and the clinician who prescribes pulmonary rehabilitation should be aware of them. Combinations of different but complementary strategies might be more beneficial than the cumulative effect of the benefits of each one.
Dynamic hyperinflation reduction has a key role in the improvement of exercise tolerance and might be achieved by combining strategies that enhance expiratory flow (e.g. pharmacological optimization with inhaled bronchodilators) with a ventilatory demand and respiratory rate reduction (e.g. through oxygen supplementation and high intensity interval training). However, it should be noted that the eventual role of oxygen supplementation beyond the effects found in laboratory studies, in exercise training of non-hypoxemic patients, particularly those that do not desaturate while exercising, has not yet been demonstrated.
Motivation in complying with medication, with an active lifestyle and regular exercise might generate positive reinforcement and could counteract the negative spiral of dyspnea, becoming sedentary, deconditioned and more dyspneic. It is well documented in the literature that there is an association between lower physical activity, the risk of exacerbation with hospitalization and mortality in patients with COPD42. Exercise training is the treatment that does most to improve and preserve the muscle mass and exercise performance, both relevant prognostic indexes in COPD.
Exercise training adaptations due to comorbiditiesAn accurate and individualized exercise prescription should necessarily include some patient adaptation due to comorbidities. The prevalence of comorbidities, particularly multiple comorbidities, reflects the growing evidence of COPD as a complex disease that impacts on multiple organic systems. Systemic inflammation enhances the risk of developing and worsening conditions like coronary heart disease, congestive heart failure, diabetes mellitus, peripheral vascular disease, muscle dysfunction, cachexia and osteoporosis, among others.43
Although they influence patient prognosis, comorbidities should not preclude indication for pulmonary rehabilitation.44 On the contrary, PR programs should be adapted to comorbidities, not only in the evaluation settings but also in the prescription of PR modalities and monitoring PR programs. Exercise training, the core of PR programs, has also demonstrated well documented benefits in several comorbidities. Moreover, exercise training has a clear and high level of evidence-based benefits. The level of recommendation is IA for conditions like coronary heart disease, congestive heart failure, diabetes mellitus and peripheral vascular disease.45,46
The most relevant exercise training adaptations in patients with comorbidities are:
- •
Coronary heart disease: a moderate intensity training is recommended, being cautious in relation to intensity progression (start with intensities of 50 to 60% of maximal heart rate attained in previous maximal exercise test and, if well tolerated, gradually increase the intensity to a maximal threshold of 80% of peak heart rate); train below the anaerobic threshold, and if ischemic or arrhythmic thresholds are present, train below those limits; train under clinical surveillance and telemetric monitoring, at least in the first few weeks.
- •
Congestive heart failure: slow progression of exercise intensity, as tolerated; start with intensity of 40% peakVO2 and progress to 80% peakVO2; clinical and telemetric monitoring and active search for signs or symptoms of instability (central or peripheral edema, rapid weight gain, pulmonary congestion) are highly recommended.
- •
Diabetes mellitus: an accurate monitoring of glucose is recommended to improve metabolic control; prevention of hypoglycemia (with diet/nutritional counseling and pharmacological adjustments, if needed); search for metabolic derangements (exercise is contraindicated if glycemia>250mg/dL with ketonuria or if glycemia>300mg/dL); be aware of possible sub-clinical coronary disease; evaluate, prescribe and monitor evolution according to this information; diabetic foot surveillance is recommended, with particular attention to adequate hydration.
- •
Peripheral vascular disease: privilege gait training above ischemic threshold for lower limbs (training elicits claudicating symptoms; if intense pain is present, training is followed by a brief period of rest to permit symptoms to resolve; repeat for 30min to 1h); it should be noted that peripheral vascular disease is frequently associated with systemic vascular disease, including cerebral and coronary, though an active search for sub-clinical coronary disease is mandatory.
Although strongly recommended by scientific societies, pulmonary rehabilitation programs still need to be more widely implemented. PR programs have shown high level of evidence of benefits in chronic respiratory patients, particularly those with COPD. Exercise training is an essential component of PR programs and its benefits are well documented in the literature. Clinicians should be aware of the different strategies available in order to optimize exercise training, patient security and compliance. Generalization and development of the existent PR programs and implementation of new strategies and modalities should be a constant preoccupation for all clinicians involved in the care of respiratory patients.
Patients with advanced COPD show significant exercise intolerance due to severe muscle dysfunction and intense dyspnea. In these patients, exercise with interval training – repeated short high intensity training periods (30s to 3min), interspersed with rest or low intensity periods, and/or global strength training or localized to quadriceps, under oxygen supplementation if needed, might constitute suitable, applicable and efficacious exercise training strategies.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.