A 28-year-old neuromuscular patient chronically treated with nocturnal noninvasive ventilation developed pulmonary lobar atelectasis and daytime hypoxemia. Twenty four-hour 5L/min oxygen was begun, while mechanical cough assist aids were applied for seven days. In the following three days, treatment with nebulized Dornase alpha (rhDNase) b.i.d. was tested, without any significant improvement. On 11 and 13th days rhDNase was instilled by flexible bronchoscopy. A rapid resolution of the atelectasis was observed with relief of hypoxemia, without significant side effects. On day 16 the patient was discharged without oxygen requirements. In non-intubated neuromuscular patients with atelectasis who do not respond successfully to non-invasive treatments intrabronchial instillation of rhDNase may safely help to improve airway clearance.
Um doente neuromuscular crónico de 28 anos de idade, tratado com ventilação noturna não invasiva, desenvolveu atelectasia lobar pulmonar e hipoxemia diurna. Foi iniciado suporte de oxigénio durante 24 horas, enquanto uma ajuda mecânica para a tosse era aplicada por 7 dias. Nos 3 dias seguintes o tratamento com Dornase alfa nebulizado (rgDNase) b.i.d. foi testado, sem qualquer melhoria significativa. No 11.° e 13.° dias rhDNase foi introduzido por broncoscopia flexível. Um restabelecimento rápido da atelectasia foi observado com alívio da hipoxemia, sem efeitos secundários significativos. No 16.° dia o doente teve alta sem necessidade de oxigénio. Em doentes neuromusculares não intubados, com atelectasia, que não respondam positivamente a tratamentos não invasivos, a introdução intrabronquial de rhDNase pode com segurança ajudar a melhorar a abertura das vias respiratórias.
In neuromuscular disease, impairment of cough mechanisms due to expiratory muscle weakness can favor the development of atelectasis after pulmonary infections.1 This can lead to hypoxia with a life-threatening clinical situation. There is a lack of evidence-based studies on the management of infectious atelectasis.2 Although chest physiotherapy, mechanical in-exsufflation (MI-E) and high-frequency chest wall oscillation (HFCWO) improve airway clearance,3–6 they may not be sufficient, particularly when secretions become highly viscous due to accumulation of significant amounts of extracellular DNA.
Recombinant human Dornase alpha (rhDNase) may cleave and depolymerize extracellular DNA, and separate it from proteins: this allows endogenous proteolytic enzymes to break proteins and decrease viscoelasticity and surface tension of purulent sputum. In patients with cystic fibrosis rhDNase has proved extremely effective, with both aerosol administration7 and bronchoscopic instillation.8 Studies in newborns or in children have reported beneficial effects of rhDNase on atelectasis in non-cystic fibrosis patients,9,10 and anecdotal reports have suggested a beneficial effect in respiratory syncytial virus bronchiolitis11 and in atelectasis due to mucus plugs in newborns and children.12
Most of these studies were performed by instillating the drug directly into the trachea in intubated patients. Although most patients showed an improvement within 24h, in some of them direct instillation resulted in clinical deterioration, presumably due to a mucus mobilization that was too rapid.10 This effect may be dangerous in neuromuscular patients if means for removal of the mucus are not immediately available, or if the rate of mobilization exceeds capacity of elimination. However, no studies reporting effects of rhDNase in neuromuscular patients have been published. In this article we describe the favorable clinical course of a non-intubated neuromuscular patient with infectious atelectasis treated with bronchoscopic instillation of rhDNase.
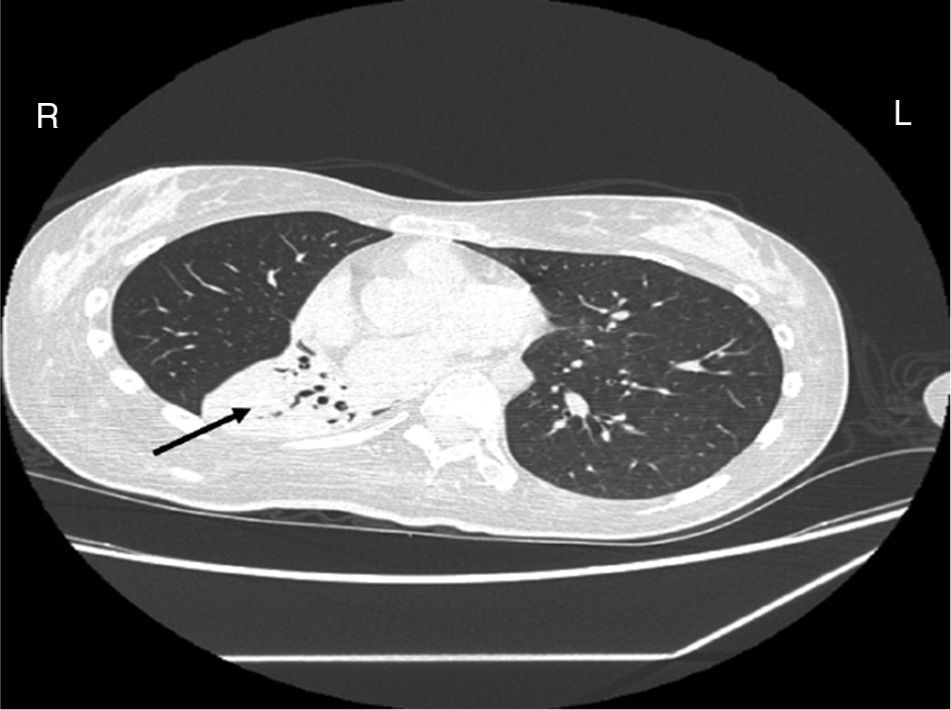
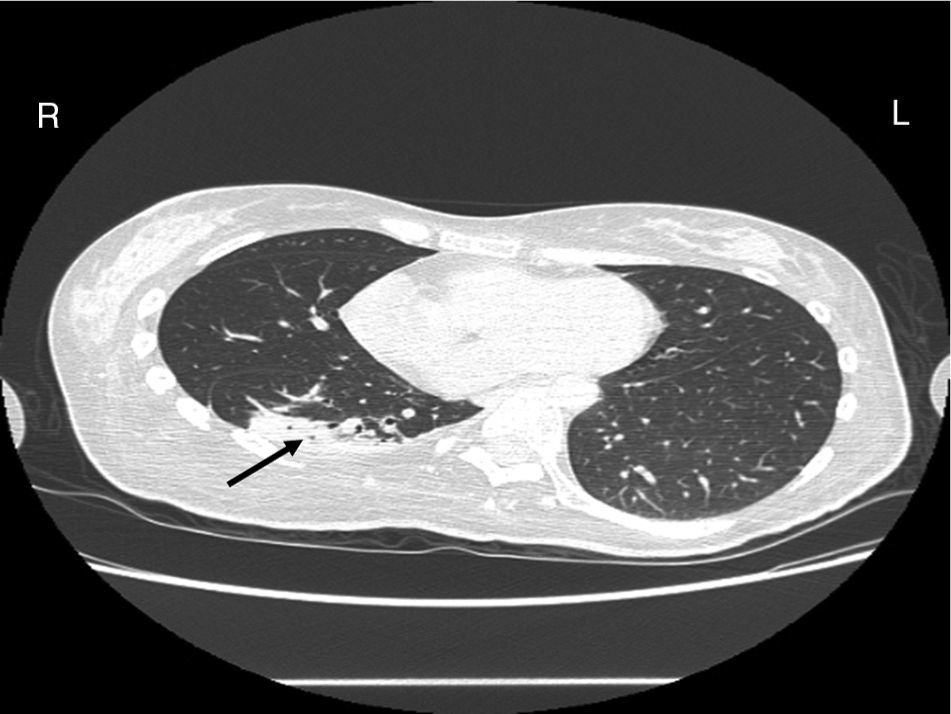
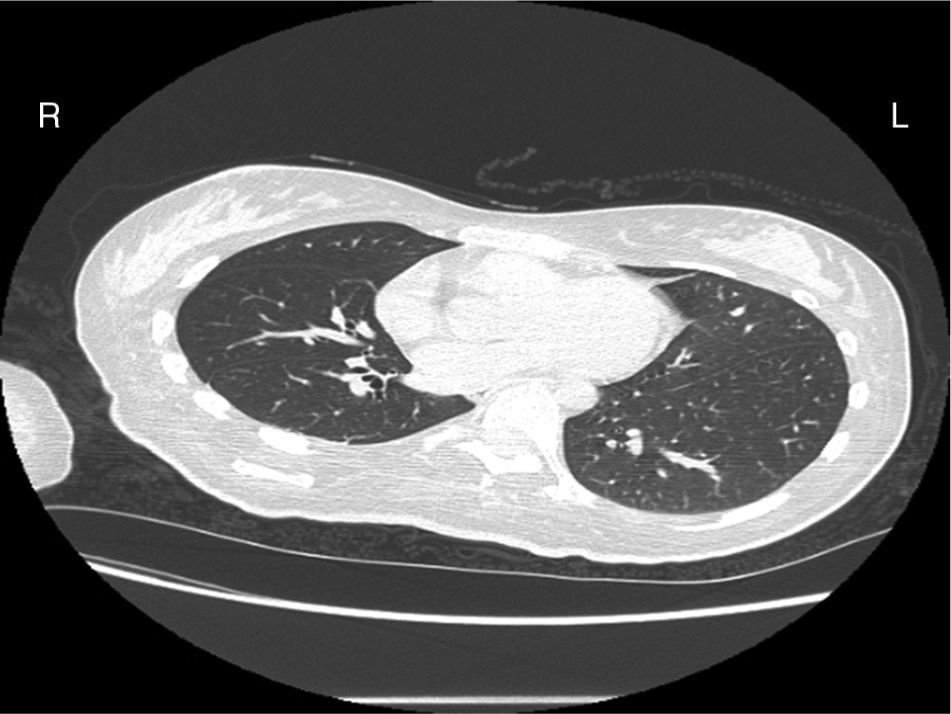
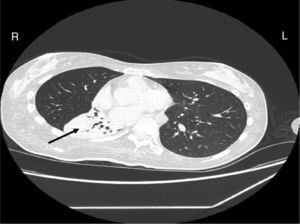
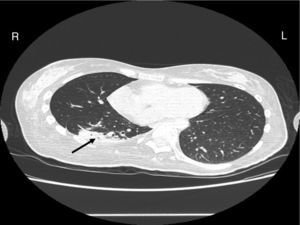
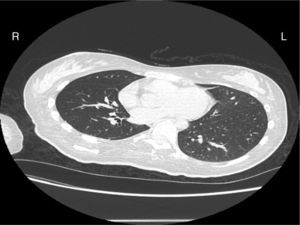
Case reportA female patient with a congenital muscular dystrophy had been treated with nocturnal nasal noninvasive positive pressure ventilation (NIV) since she was 20. At the age of 26 she was hospitalised for a complete atelectasis of the right lower lobe, and had recovered after more than a month with the application of an intensive combined protocol of HFCWO (The Vest Airway Clearance System, Hill-Rom St. Paul, MN, USA) plus manual and mechanical assist cough (In-Exsufflator, Cough-Assist®, Philips Respironics, Murrysville, PA, USA). Her clinical condition remained good until the age of 28, when her lung function tests showed the following values: vital capacity 0.55 L (16% of the predicted value), maximal inspiratory pressure 10cmH2O (11% of predicted), maximal expiratory pressure 14cmH2O (13% of predicted), and peak cough expiratory flow 80L/min. A few months later, due to the appearance of fever and copious mucus production, she was treated with manual and mechanical chest physiotherapy plus antibiotics (ceftriaxone) initially. However, she still complained of dyspnea and a feeling of retained secretions, and was then admitted to hospital. She had a severe left convex scoliosis with a mean Cobb angle of over 70°. After a chest X-ray, a CT scan was performed (Fig. 1) which showed atelectasis of the right lower lobe. Her diurnal arterial oxygen saturation (SpO2) fluctuated between 82 and 85% in room air, while PaCO2 was normal. The patients showed minimal clinical signs of dehydration, namely dry mouth. She had good skin turgor and normal urine output. Haematocrit and electrolytes were normal except for potassium which was lower than normal. We began nutritional support and hydration because the patient was not able to eat and drink enough. Negative results were obtained from sputum cultures; however, intravenous antibiotics were administered. Twenty-four-hour NIV was begun, with the addition of oxygen (5L/min), as NIV alone was not enough to maintain SpO2 above 90%. Fifteen-twenty minutes sessions of HFCWO at a pressure of 5cm H2O and a frequency of 12Hz were performed; each session was followed by five or six sessions of mechanical assist cough with an In-Exsufflator at pressures of +40/−45cm H2O, delivered respectively over 3 and over 2s, with an abdominal thrust timed to the exsufflation cycle. This protocol was applied 4 times/day; additionally, In-Exsufflator was used on demand. Once 24-h NIV had begun, we asked the patient to frequently change her decubitus. However, as she had a severe scoliosis, she hardly changed her body position tending to remain on her right side. As the patient demonstrated a modest clinical improvement, we hypothesized that bronchial secretions had a high concentration of DNA due to accumulation of degenerated leukocytes. Therefore, one week after admission we tested rhDNase (Pulmozyme®; Roche, Basel, Switzerland). For three days, 2.5mg were delivered twice daily with a jet nebulizer, using an in-line nebulizer with NIV, without success. The next day flexible fiberoptic bronchoscopy (FOB) was performed, during NIV plus oxygen, to get a better evaluation of the cause of the obstruction and to possibly instill rhDNase bronchoscopically. FOB showed a lot of very thick mucus in the lower right lobe, but, due to its high viscosity, only a small amount could be removed. Then, a single dose of 2.5mg rhDNase was instilled directly over the affected area. As the procedure was performed in a clinical ward and the patient was at risk of intubation, this was carried out together with an Intensivist. Anyway, we had no complications except for a mild transient decrease in SpO2. The patient was closely monitored by trained nurses. Copious but thinner secretions were removed with the help of the In-Exsufflator. It had to be used six times in the first hour and three times in the second hour for periods lasting from 1 to 2min to 10 or more consecutive minutes. In the following hours the In-Exsufflator was applied only according to the protocol and within 24h a significant clinical improvement was evident, with a reduced need of oxygen. A new CT scan demonstrated a significant reduction of the atelectatic area (Fig. 2). The treatment was repeated two days after the first instillation with the same dose of the drug, with a further clinical improvement that allowed us to withdraw oxygen. Even after the second instillation, MI-E was often required, four times in the first hour and once in the second hour, unlike in the following days when it was applied only according the protocol. Two days later the patient was discharged (Table 1). A month later the clinical and radiological recovery was confirmed (Fig. 3).
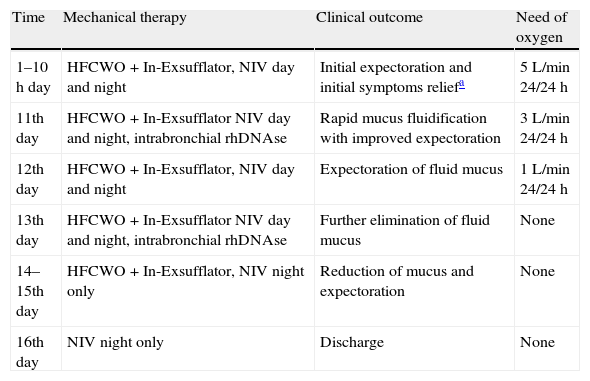
Relationship of therapeutic measures with clinical outcomes and oxygen need.
| Time | Mechanical therapy | Clinical outcome | Need of oxygen |
| 1–10h day | HFCWO+In-Exsufflator, NIV day and night | Initial expectoration and initial symptoms reliefa | 5L/min 24/24h |
| 11th day | HFCWO+In-Exsufflator NIV day and night, intrabronchial rhDNAse | Rapid mucus fluidification with improved expectoration | 3L/min 24/24h |
| 12th day | HFCWO+In-Exsufflator, NIV day and night | Expectoration of fluid mucus | 1L/min 24/24h |
| 13th day | HFCWO+In-Exsufflator NIV day and night, intrabronchial rhDNAse | Further elimination of fluid mucus | None |
| 14–15th day | HFCWO+In-Exsufflator, NIV night only | Reduction of mucus and expectoration | None |
| 16th day | NIV night only | Discharge | None |
This experience illustrates the safety and efficacy of intrabronchial rhDNase treatment in the management of infectious atelectasis in a non-invasively ventilated neuromuscular patient in whom physiotherapy techniques alone did not produce a rapid significant improvement.
To the best of our knowledge, to date rhDNase has not been tested in neuromuscular patients. We initially used the drug in a nebulized form, and switched to intrabronchial instillation after that it was unsuccessful. Direct intratracheal instillation of the drug appeared to be far more effective than its inhalation. Actually in patients on mechanical ventilation nebulized administration of the drug may result in a significant deposition in the ventilator tubing. As we continued our usual physiotherapy treatment protocol after the rhDNase administration, we cannot confirm that the drug caused the patient's recovery from atelectasis; however, a significant clinical improvement rapidly started only after the drug instillation. Interestingly, the same temporal relationship between drug administration and clinical improvement has been observed in the other clinical series.8,9 In common clinical practice FOB itself is considered a possible way of facilitating mucus mobilization.13 However, a small randomized controlled trial showed that it is no better than physiotherapy for resolving lung volume loss.14 In addition, atelectasis frequently recurs after bronchoscopy. Conversely, since the drug was instilled our patient easily expelled her secretions and progressively improved with no side effects apart from a mild and short-lived decrease in SpO2. This treatment was well tolerated by the patient who was not willing to undergo a long hospitalization.
Best clinical practice in neuromuscular patients usually requires non-invasive means to manage respiratory complications; however, intrabronchial instillation of rhDNase could be a supplementary method of treatment. As rhDNase is an expensive drug, a cost-benefit assessment would be warranted. We cannot accurately estimate how long the hospital stay would have been for our patient if rhDNase had not been used. However, compared to the previous episode of atelectasis, we saved 19 days of hospitalization. One vial of the drug costs 43 euros, and two vials were used for local instillation. Since one day's stay in our hospital costs 350 euros, the drug would have been cost-effective even if the stay were reduced by just one day.
In conclusion, treatment with rhDNAse demonstrated a safe option for this neuromuscular patient with atelectasis who had not responded to normal intensive treatment. Bronchoscopic instillation gave the best results, liquefying secretions that were easily eliminated with the In-Exsufflator. As persistent atelectasis may be a life-threatening condition in neuromuscular patients, we suggest that this drug should be considered for neuromuscular patients when appropriate. Further studies are needed in order to confirm its efficacy and the best method of administration.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank the components of our nursing staff for their dedication, especially V. Aiello and B. Di Simone. We also thank F. Greco and the Italian Union against Muscular Dystrophy (U.I.L.D.M.) of Palermo for their support of the patient and her family.