The incidence of pregnancy-associated cancer is relatively low, complicating only 0.02–0.1% of all pregnancies. The authors describe a case of a 36-year-old woman, a light smoker, who was admitted to the hospital at 27 weeks of pregnancy, with respiratory symptoms since second trimester. Chest X-ray showed total left lung opacity with contralateral mediastinal deviation, suggestive of pleural effusion, and the pleural biopsy revealed invasion by lung adenocarcinoma. EGFR mutation test was negative. After a multidisciplinary meeting, it was decided to start fetal lung maturation and cesarean section at 29 weeks gestation. The patient received two lines of chemotherapy and bone metastasis radiotherapy, but there was progression of the disease. An EML4-ALK translocation was identified in an additional genetic test. Crizotinib 250mg BID was started. The patient showed a progression-free survival of 9 months and died 19 months after lung adenocarcinoma was diagnosed.
A incidência de neoplasia associada à gravidez é relativamente baixa, podendo atingir cerca de 0,02-0,1% de todas as gestações. Os autores descrevem o caso de uma gestante de 36 anos de idade, fumadora, admitida no hospital às 27 semanas de gestação com sintomas respiratórios desde o segundo trimestre. A telerradiografia do tórax mostrou opacidade total do pulmão esquerdo, com desvio contralateral do mediastino, sugestiva de derrame pleural e a biópsia pleural revelou invasão por adenocarcinoma pulmonar. A pesquisa da mutação EGFR foi negativa. Após reunião multidisciplinar, decidiu-se iniciar a maturação pulmonar fetal e cesariana às 29 semanas de gestação. A doente realizou 2 linhas de quimioterapia bem como radioterapia óssea paliativa, verificando-se progressão da doença. A translocação EML4-ALK foi identificada num teste genético adicional. Foi iniciado crizotinib 250mg 2x dia. A doente apresentou uma sobrevida livre de progressão de 9 meses e faleceu 19 meses após o diagnóstico.
Lung cancer has overtaken breast cancer as the leading cause of cancer death in women and the incidence rate has been increasing.1,2 In 2010, data from USA estimated 105770 new cases of lung cancer and 71080 cases of deaths among women.1 Only 1–6% of patients with lung cancer are younger than 40 years old and the proportion of female patient is about 24–46%.3
The incidence of pregnancy-associated cancer is relatively low, complicating only 0.02–0.1% of all pregnancies.4 Current trends to delay pregnancy and the age-dependent increase in the incidence of several malignancies are expected to raise the occurrence of pregnancy-associated cancer.3,4
The association of lung cancer and pregnancy has rarely been described. Few more than 40 cases have been reported in the literature, 77% were non-small cell carcinoma (NSCLC) and most of them were adenocarcinoma.5 In the majority of cases, patients were diagnosed with advanced disease not amenable to resection and requiring systemic treatment.3,5 Post-partum maternal median survival is generally poor and the majority was known to have died within 1 year after delivery.5 Metastatic transmission to the products of conception is a rare phenomenon, but 11 cases of placental metastases and 3 cases of fetal metastases were reported secondary to maternal lung cancer.3–6
There is not enough information to establish a conclusion about the security of chemotherapy or targeted agents during pregnancy. There have been frequent reports of anticipation of delivery, especially in women with advanced disease and poor prognosis.4,7,8
EML4-ALK mutation in NSCLCThe EML4-ALK fusion oncogene represents one of the newest molecular targets in NSCLC and defines a new molecular subset with distinct clinical and pathologic features.9,10 The patients most likely to harbor EML4-ALK are young, never/light smokers with adenocarcinoma.9–11 These patients share many of the clinical features of NSCLC patients likely to harbor EGFR mutation. However, apart from rare exceptions, EML4-ALK and EGFR mutation are mutually exclusive.12,13
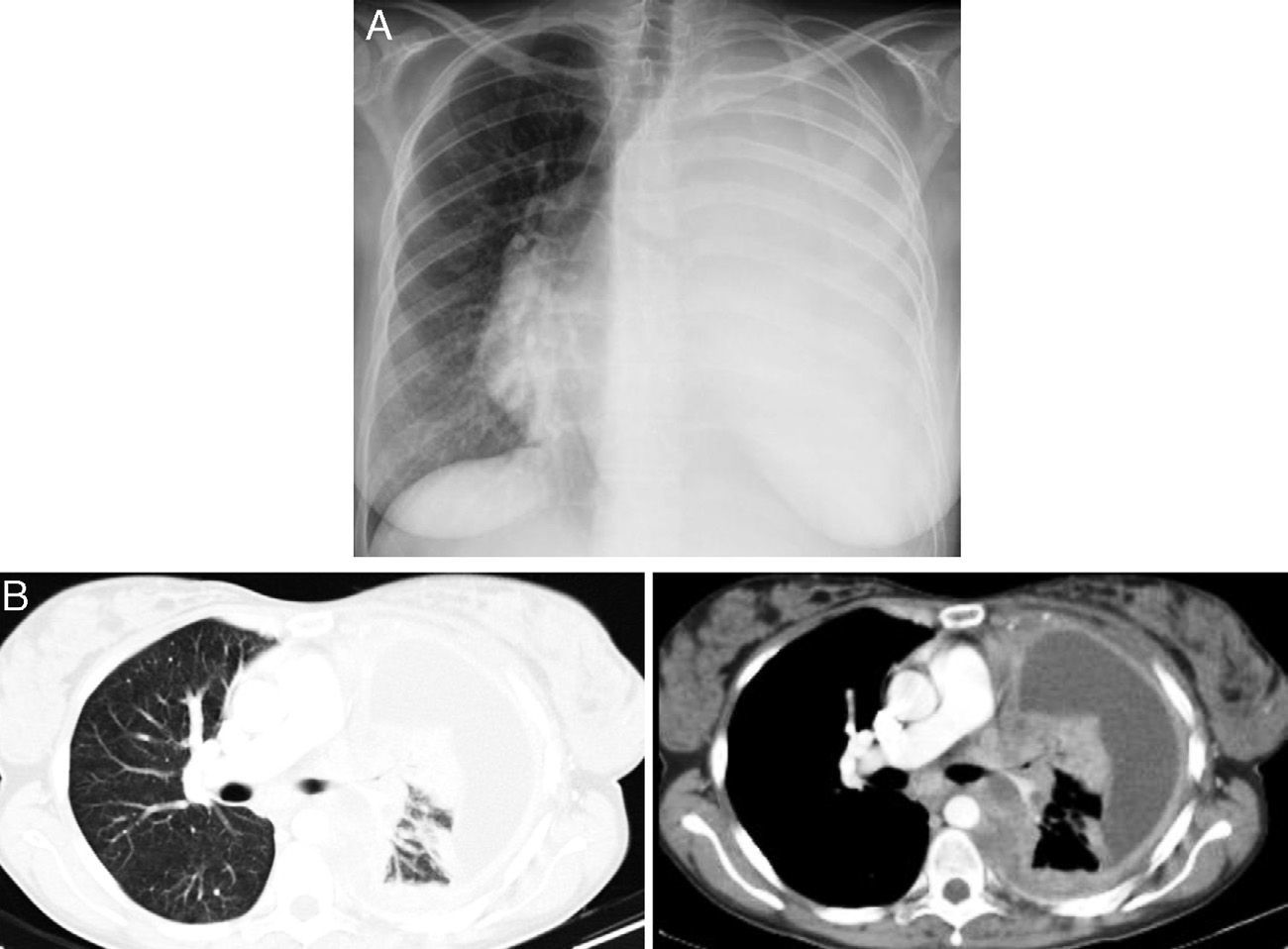
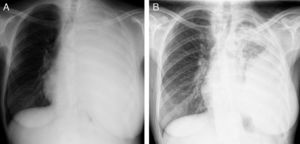
Case reportA 36-year-old light-smoking pregnant woman was admitted to hospital at 27 weeks gestation in August 2010 with a history of malaise, fatigue, exertional dyspnea, dry cough and left pleuritic chest pain since the second trimester of the gestation. No regular medication was reported. On physical examination, she appeared to have poor performance status (PS 3). Pulmonary auscultation revealed reduction of the breath sounds in the left lung field. Chest X-ray showed total left lung opacity with contralateral mediastinal deviation, suggestive of pleural effusion (Fig. 1A). A thoracocentesis and pleural biopsy were performed revealing the diagnosis of pleural invasion by lung adenocarcinoma. The immunohistochemical study showed CK7, CK8/18 and was napsin positive and negative for CK20, estrogens and progesterone receptors. The test for EGFR mutation (direct DNA sequencing) was negative.
After a multidisciplinary meeting (Pulmonology, Obstetrics and Neonatology), the decision was made to start fetal lung maturation and cesarean section at 29 weeks gestation. The female newborn had an Apgar index 8/9 and weighed about 1.2kg. Placental pathological examination showed no malignancy involvement. The newborn was discharged home from Neonatology Department by the 47th day and nowadays she is a healthy female child.
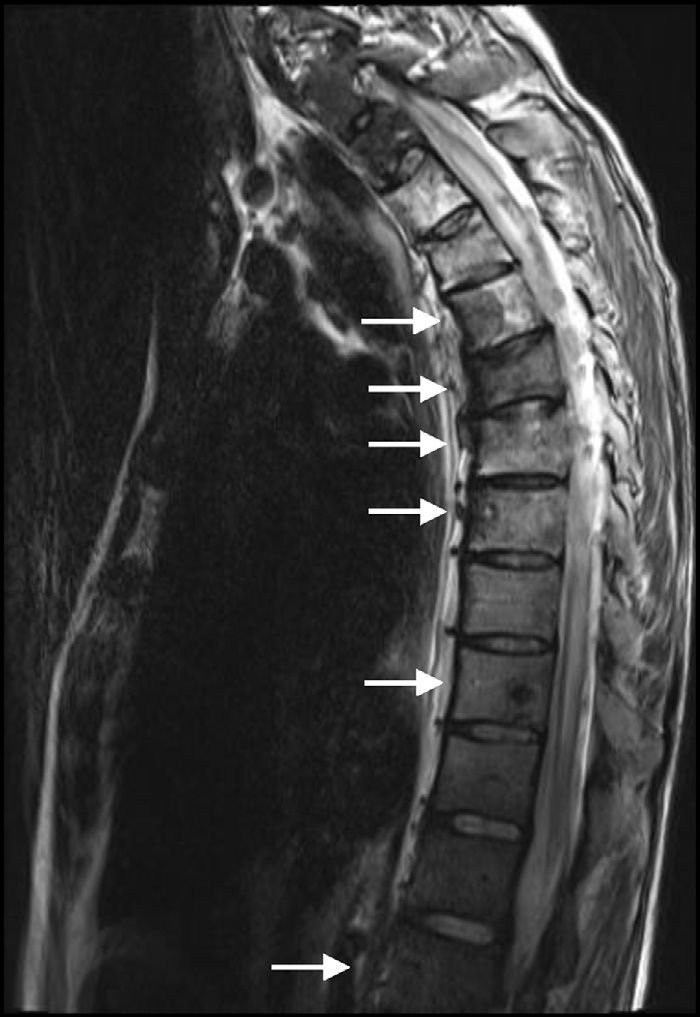
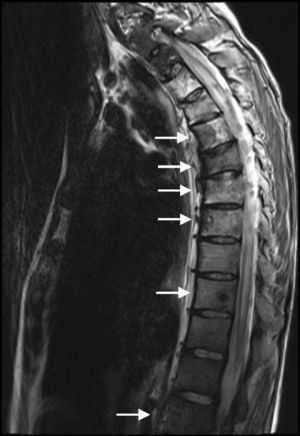
After delivery, the patient underwent a thoracoabdominal CT which depicted a large left hilar mass with no plane of cleavage with vascular structures, multiple implants on mediastinal pleura, and a large volume of left pleural effusion (Fig. 1B), and multiple bone and liver metastases. First line chemotherapy with carboplatin plus oral vinorelbine (AUC 5 and 60mg/m2, respectively) was started on September 2010. Intravenous zoledronic acid was also prescribed. After 4 sessions of chemotherapy, despite some clinical improvement, there was progression of the disease with the spreading of bone disease and neurological symptoms of paraplegia and sphincter disruption (Fig. 2). Subsequently, the patient underwent second line chemotherapy with pemetrexed (500mg/m2), and thoracic spine (T4–T10) radiotherapy (30Gy/12 sessions), but without clinical efficacy.
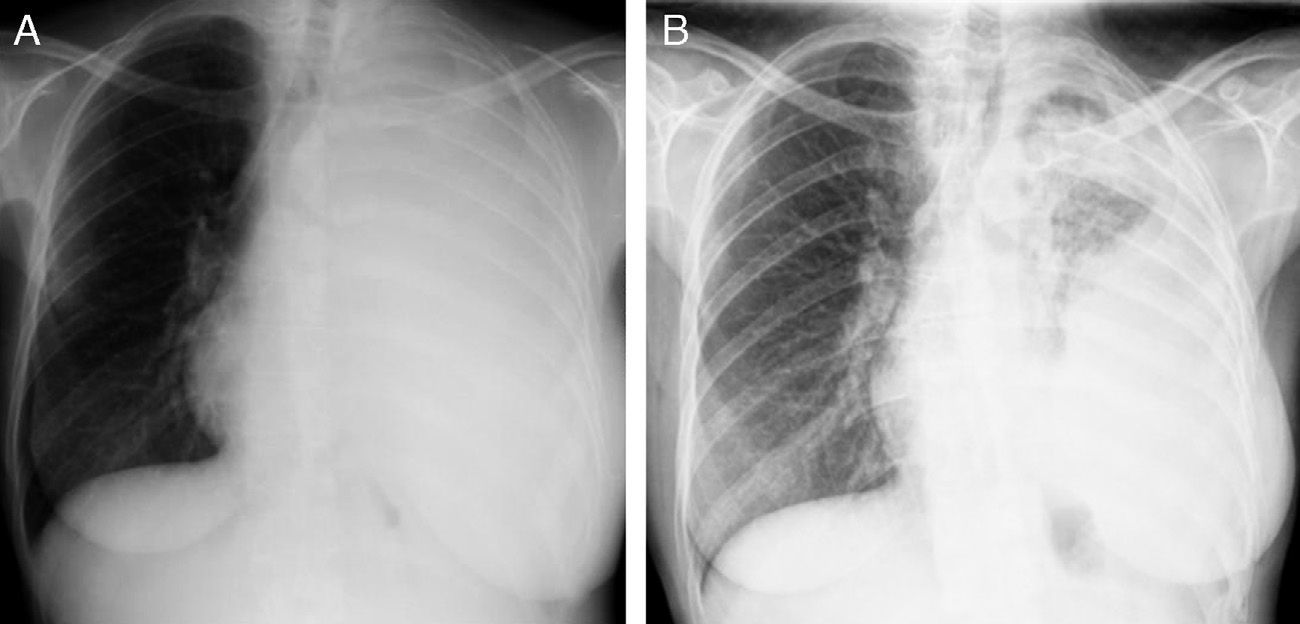
An EML4-ALK translocation (FISH method) was then identified in an additional genetic test and crizotinib 250mg BID was started on March 2011. The patient showed global symptom relief and improvement of neurological signs, and also partial radiological response (Fig. 3) based on RECIST criteria. She showed only mildly elevated hepatic transaminases, without other adverse effects. Her progression-free survival was 9 months and she died 19 months after diagnosis.
DiscussionThere are only a few cases in the literature describing the diagnosis of lung cancer during the course of a pregnancy. The median diagnosis onset age reported is around 34–36 years, with a median gestational age at diagnosis of 27–29 weeks. Although the clinical picture of lung cancer is similar in pregnant and non-pregnant patients, a pregnant woman is more likely to be diagnosed with more advanced or metastatic disease.14
During pregnancy, there is a general concern about performing the examinations required for cancer diagnosis. This fact, in addition to the nonspecific nature of the symptoms, can delay diagnosis. It is important to be aware that small biopsies can be performed during pregnancy and usually without harming the mother or fetus.
In most types of cancer the histopathological features are similar in both pregnant and non-pregnant women.4 NSCLC is the most frequent lung cancer diagnosed and adenocarcinoma the predominant histological subtype.5
Treatment decisions related to lung cancer in a pregnant woman are a complex emotional and ethical challenge. This decision can be more difficult when the cancer is at an advanced stage at diagnosis and there is no effective treatment available. Cancer diagnosis during pregnancy is a clinical challenge with conflicting views as to whether to immediately start treatment at diagnosis or terminate the pregnancy earlier.
Given the very limited data available regarding the safety of different chemotherapeutics and targeted therapies during pregnancy, and because of the small number of patients treated, it is very hard to establish a standardized approach to manage these cases.4 As soon as the pulmonary maturation of fetus is completed, it is advisable to proceed with delivery to permit the beginning of maternal chemotherapy. Although vertical transmission of cancer cell to the placenta or the fetus is exceptionally uncommon, in some cases treatment of the mother during pregnancy might also be considered.
From the oncologist's point of view, termination of pregnancy would be the best choice in order to focus on the treatment of the mother. From the obstetrician's point of view, early start of the mother's treatment could prevent metastases in the fetus and provide more time for pregnancy development. A multidisciplinary approach is mandatory, as was used in the case described; all the specialists involved contributed with their experience and gave reasoned opinions about the case.
In our patient the diagnosis was performed at 27 weeks gestation. Our decision to prolong pregnancy for 2 more weeks and start fetus pulmonary maturation at that point provided the possibility of avoiding the risk of starting chemotherapy during pregnancy.
This treatment decision did not seem to compromise the mother's survival. Most reports of lung cancer during pregnancy described a survival rate lower than 1 year.7
Although it was an initial advanced stage IV disease, the patient survived 19 months after delivery. The EML4-ALK mutation testing was crucial and after starting third-line crizotinib, the patient had a progression-free survival of 9 months, similar to the median progression-free survival described in the recent phase 1 clinical trial.15 Studies carried out so far show that, in patients with advanced EML4-ALK-positive NSCLC, crizotinib therapy seemed to be associated with improved survival.15–17
In conclusion, no exact solution is possible for cases of cancer treatment during the course of pregnancy. We may help future decisions by sharing diverse perspectives and treatment managements in case reports.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Neves I, Mota PC, Hespanhol VP. Cancro do pulmão durante a gravidez: um caso incomum. Rev Port Pneumol. 2014;20:46–49.