AbbreviationsATS – American Thoracic Society –Fractional exhaled nitric oxide – Global Initiative for Asthma –Inhaled corticosteroids –Immunoglobin E – National Institute for Health and Care Excellence
Asthma is one of the most common chronic diseases, causing significant burden to healthcare systems and societies.1 The prevalence of asthma is increasing in many countries, especially among children. In Portugal, asthma affects 6,8% of the population.2
Severe asthma, according to the Global Initiative for Asthma (GINA), is a subset of difficult-to-treat asthma, defined as “asthma that is uncontrolled despite adherence with maximal optimized therapy and treatment of contributory factors, or that worsens when high dose treatment is decreased”.3,4 Severe asthma affects approximately 5–10% of asthma patients and is associated with increased morbidity, risk of hospitalization from exacerbations, mortality, and related healthcare costs.5
Type 2 inflammation-driven asthma (type 2 asthma) is mediated by type 2 cytokines (e.g. IL-4, IL-5, IL-13) and is very heterogeneous in nature.3 Type 2 asthma is present in most asthmatic children and about 50% of asthmatic adults.6,7 There is a high proportion of type 2 asthma among patients with severe asthma. Type 2 asthma is usually associated with increased eosinophilic inflammation, raised serum immunoglobin E (IgE) and raised fractional exhaled nitric oxide (FeNO) levels. Because Type 2 inflammation results from complex pathophysiologic mechanisms involving both innate and cellular immunity, the criteria to define type 2 asthma is still a matter of debate. According to GINA, either refractory or underlying type 2 inflammation should be considered if: (i) blood eosinophils ≥150/μl, and/or (ii) FeNO ≥20ppb, and/or (iii) sputum eosinophils ≥2%, and/or (iv) asthma is clinically allergen-driven.3
All guidelines recommend the use of biomarkers, such as FeNO, IgE or blood eosinophil counts, to characterize severe asthma, although there is still disagreement regarding the cut-off values.3,8,9 Biomarkers are important tools to classify asthma patients into phenotypes. Asthma phenotypes do not define asthma severity, as the correlation between biomarker levels and disease severity is limited, and, for some uncontrolled patients, there are no detectable changes in biomarker levels. However, biomarker-based phenotyping of asthma patients, in association with detailed clinical evaluation, is essential for the proper characterization of the patient and to define the most appropriate therapeutic strategy. This is particularly important considering the high proportion of uncontrolled asthma patients in many countries, including Portugal.
Nitric oxide is a key modulator of immune responses and is overproduced in the airways of type 2 asthma patients. FeNO levels have long been recognized as a valid biomarker for the clinical management of severe asthma, not only for the phenotypic characterization of the disease, but also for assessing the disease evolution and treatment strategies.3,8,10 FeNO levels are particularly useful as a diagnostic tool in ICS-naïve patients, to monitor patient compliance with treatment, and are good predictors of response to ICS, asthma exacerbations and decline of lung function.11 In particular, the combination of elevated FeNO levels and an elevated blood eosinophil count is a good indicator of the higher exacerbation burden in severe asthma.12 FeNO levels are also useful to monitor and guide the therapeutic strategy, and to predict the responsiveness to some biological therapies.13 In short, FeNO evaluation is a low-cost, non-invasive procedure that is useful as a surrogate biomarker for the assessment and management of severe asthma. Therefore, the routine use of FeNO testing can play an important role in effective asthma control.
Interpreting FeNO levels is not straightforward as there are many possible underlying causes for variations in FeNO levels: smoking habits, diet, respiratory tract infections, time of day, height, gender, age, etc.11 This precludes the use of this biomarker alone to define type 2 severe asthma and most specialists rely on several biomarkers simultaneously in endotype assessment. FeNO levels are easier to interpret when very high levels are observed.
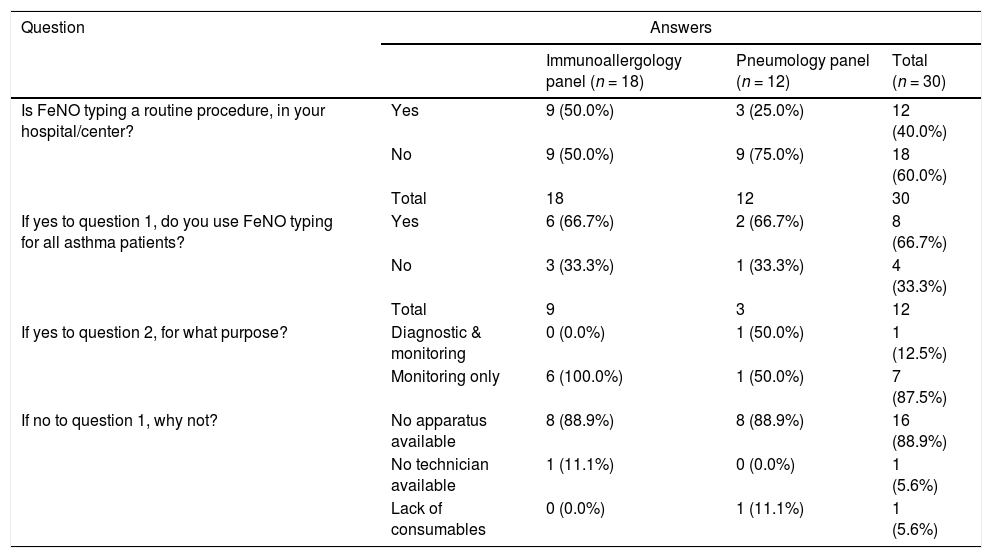
In Portugal, biomarker-based asthma phenotyping assays are reimbursed by the public national health service. However, FeNO testing is not reimbursed by private insurance companies. In addition, FeNO testing is not available at all hospitals/centers. In order to assess clinical practice of FeNO testing in Portugal, a panel of immunoallergologists and pulmonologists was informally invited to participate anonymously in an online survey. The data obtained (see Table 1), demonstrates that FeNO testing is still not routinely used in clinical practice in Portugal mainly due to access constraints: the lack of devices or consumables for FeNO measurement. Interestingly, for those who reported using FeNO testing with all asthma patients, the biomarker is mainly used for disease activity monitoring purposes, and for those who use FeNO routinely but not for all patients, the reported criteria for testing included severe and type 2 asthma patients.
Summary of the answers to the online survey regarding FeNO testing clinical practice in Portugal.
Note: A panel of immunoallergologists and pulmonologists was informally invited to answer an online survey in order to assess the clinical practice of FeNO testing in Portugal; a total of 30 valid answers were obtained.
In conclusion, although FeNO testing appears to be recognized by Portuguese asthma specialists as an important tool for disease management, particularly in patients with severe and/or type 2 asthma, there are access constraints to this assay that need to be addressed in Portugal. Generalized access to FeNO testing will add value to the clinical management of asthma patients and improve symptom control. FeNO testing is also particularly relevant in severe asthma patients as it can serve to predict responsiveness to some new biological therapies and contribute to more cost-effective therapeutic options: e.g., high FeNO levels are one of the prescription criteria for dupilumab, a novel biologic for the treatment of severe asthma. We thus recommend that FeNO testing should be established as a routine procedure in clinical practice in Portugal for the diagnosis and management of asthma patients or at least be available in all reference centers.