In the conventional management of the morbidly obese that normalizes the apnea-hypopnea index (AHI), CO2 levels often remain elevated.
MethodsA retrospective review of morbidly obese patients using volume preset settings up to 1800ml to positive inspiratory pressures (PIPs) of 25–55cm H2O, or pressure control at 25–50cm H2O pressure via noninvasive interfaces up to continuously (CNVS).
ResultsTwenty-six patients, mean 55.6±14.8 years of age, weight 108–229kg, mean BMI 56.1 (35.5–77)kg/m2, mean AHI 69.0±24.9, depended on up to CNVS for 3 weeks to up to 66 years. There were eleven extubations and seven decannulations to CNVS despite failure to pass spontaneous breathing trials. Thirteen were CNVS dependent for 92.2 patient-years with little to no ventilator free breathing ability (VFBA). Six used NVS from 10 to 23h a day, and others only for sleep. Fifteen patients with cough peak flows (CPF) less than 270L/m had access to mechanical insufflation-exsufflation (MIE) in the peri-extubation/decannulation period and long-term. The daytime end-tidal (Et)CO2 of 14 who were placed on sleep NVS without extubation or decannulation to it decreased from mean EtCO2 61.0±9.3–38.5±3.6mm Hg and AHI normalized to 2.2. Blood gas levels were normal while using NVS/CNVS. Pre-intubation PaCO2 levels, when measured, were as high as 183mm Hg before extubation to CNVS.
ConclusionsVentilator unweanable morbidly obese patients can be safely extubated/decannulated and maintained indefinitely using up to CNVS rather than resort to tracheotomies.
Morbid obesity is defined by having a body mass index (BMI) of 40kg/m2 or more, and is associated with a number of comorbid conditions which can adversely impact survival. Increased work of breathing and ‘resetting’ of hypothalamic respiratory drive can result in hypoventilation and in cor pulmonale.1,2 Morbidly obese individuals, with or without complicating conditions, can become continuously ventilator dependent, that is, dependent on noninvasive ventilatory support (CNVS) or on tracheostomy mechanical ventilation (CTMV).
“NIV” has come to be synonymous with continuous positive airway pressure (CPAP) and bi-level PAP at spans that can normalize AHI without providing full NVS to normalize CO2 and optimally rest inspiratory muscles.2 Bi-level PAP became available in 1990 and often better normalized AHIs than CPAP but it has not been used at full ventilatory support settings aimed at normalization of CO2 in patients with ventilatory pump failure.3 Mokhlesi et al. reported that eight of 34 patients (23%) who used sleep bi-level PAP did not have a significant improvement in their PaCO2 despite normalization of their AHIs from 44±45.4 Likewise, Bouloukaki et al.5 evaluated CPAP and typical bi-level therapy and reported that around 20% of individuals had a CO2>45mm Hg after two years of therapy. Greater than the usual bi-level spans can be needed to normalize CO2.
The positive inspiratory pressures (PIPs) of mechanical ventilation during general anesthesia and neuromuscular blockade for patients with normal BMI are 17–25cm H2O as are PIPs for any patients with little or no measurable vital capacity (VC) but normal pulmonary compliance.6 However, patients with poor lung and chest wall compliance may require much higher pressures to normalize ventilation. The aim of this study is to demonstrate that NVS can at times be required to pressures over 50cm H2O for the morbidly obese to normalize CO2 levels and avoid the need for O2 therapy and tracheotomy. Ventilator dependent patients can also be extubated or decannulated to it. This study was approved by the Rutgers University Institutional Review Board as No. Pro2018001071.
MethodsThis is a retrospective study describing the use of NVS with high PIPs for morbidly obese patients presenting to two NVS centers over a 12year period who required NVS support due to ongoing hypercapnic respiratory failure despite NIV use. Patients 1–17 presented to Center A and patients 18–26 to Center B. Vital capacity (VC) (measured to correlate with weight but not reported here), cough peak flows (CPF), End-tidal (Et)CO2, and oximetry were measured at every visit Thirteen of the 17 patients of center A underwent bariatric surgery but subsequently regained weight. All were offered ketogenic diets and exercise programs, but none continued these therapies long-term. Introduction of NVS was indicated by symptomatic hypoventilation with decreased VC.
The therapeutic goals were: (1) normalization of PaCO2 and/or end-tidal carbon dioxide (EtCO2) or transcutaneous CO2 (TcCO2) tensions and oxyhemoglobin saturation (SpO2) during wakefulness and sleep to relieve symptoms of hypoventilation; (2) to extubate and/or decannulate those failing ventilator weaning parameters and spontaneous breathing trials to CNVS.
We define NVS as the use of portable ventilators, volume or pressure preset or bi-level machines, at at least full ventilatory support settings to normalize CO2 levels as opposed to “NIV” settings to only normalize AHI. Although volumes were initially prescribed for the patient to choose over a range from 700 to 1500ml, one patient increased his to 1800ml. Pressure support/control for drive pressures of 20–55cm H2O were used for patients with abdominal distension. The goal was to normalize PaCO2 around the clock. Patients used sleep-only NVS, sleep plus daytime NVS for up to 23h/day, or CNVS with little or no VFBA.
All patients were prescribed NVS, which was preferentially volume preset with physiologic back-up rates, that is, normal respiratory rates for age or about 12−16. Portable ventilators were used with active ventilator circuits with or without minimal EPAP/PEEP. Volume preset was preferred since lung volume recruitment cannot be performed when using pressure preset ventilation.6,7 Intubated patients and those using tracheostomy mechanical ventilation (TMV) were extubated/decannulated to up to CNVS with weaning, as possible.
Sleep NVS users who continued to gain weight tended to become dyspneic upon discontinuing NVS in the morning and developed fatigue, somnolence, and dyspnea when daytime hypercapnia was associated with decreases in SpO2 below 95% during the day, especially late in the day. As previously described, increased daytime use of mouthpiece/nasal NVS at that point was facilitated by using oximetry feedback. This renormalized blood gases and relieved dyspnea. Nasal or oronasal interfaces were used for sleep, although patients who required daytime as well as sleep NVS used oronasal interfaces with straps tightly applied during sleep for a more “closed system” to maintain normal blood gases. With nasal NVS during sleep large leak resulted in difficulty maintaining adequate PIPs and normal SpO2.8 Any supplemental O2 and sedating medications were discontinued to avoid increased NVS leakage out of the mouth.9,10 Patients’ cough peak flows (CPF) were measured and when less than 270L/m mechanical insufflation-exsufflation (MIE) was made available to decrease the risk of intercurrent pneumonias.11,12
Although pre-NVS AHI measurements were by polysomnography, subsequent measurements were estimated by ventilator flow and pressure sensors that store the data for analysis on personal computers. Compliance, average tidal volume, minute ventilation, respiratory rate, leaks, percent of spontaneous inspirations, and indices of residual apnea and hypopnea were monitored during sleep. However, only SpO2 and EtCO2 or TcCO2 levels were used as indications to adjust ventilator settings and interfaces, for example, whether to use nasal or oronasal interfaces. The reliability of the AHI by ventilator software has been reported to be sufficient for monitoring subjects on long-term NIV/NVS.11,13,14
Results and outcomesThe patients’ demographics, anthropometric data, any complicating neurological conditions, and whether extubated or decannulated to CNVS or simply maintained on NVS settings following polysomnography with or without transition from low span bi-level PAP are noted in Table 1. Eighteen were female and eight male, mean age 55.6±14.8 years. Weights ranged from 108 to 229kg and BMI a mean of 61.1 (range 35.5–77)kg/m2. Twenty presented with symptomatic hypercapnia or were intubated, ventilator unweanable, and wanted extubation to CNVS and MIE rather than tracheotomy and six presented using tracheostomy mechanical ventilation (TMV) (Table 1).
Demographic Data, Extubations and Decannulations for Patients with Morbid Obesity.
| Case | Age | Sex | BMI | Complicating conditions | BiPAP to intubation | Required Extubation/Decannulation to CNVS |
|---|---|---|---|---|---|---|
| 1a | 71 | M | 77 | None | 1 year 3 intubations | Extubation & Decannulation |
| 2a | 24 | M | 54.4 | Spina bífida paraplegia | 2 years 3 intubations | No |
| 3 | 43 | F | 41.4 | None | NVS to intubation | Extubation x 2 |
| 4 | 72 | F | 43.9 | Post-poliomyelitis | No | Extubation to CNVS for Surgery |
| 5a | 32 | F | 75 | None | 2 years 2 intubations | Extubation & Decannulation |
| 6a | 59 | F | 57.5 | None | 2 years 2 intubations | Extubation & Decannulation |
| 7 | 71 | M | 35.5 | Diabetic neuropathy | No | Extubation |
| 8a | 53 | F | 53.1 | None | 1 intubation | Extubation x 2 & Decannulation |
| 9a | 62 | F | 48.1 | None | 14 years 2 intubations | Decannulation |
| 10a | 81 | F | 54 | None | 8 years | No |
| 11a | 59 | F | 46.3 | None | 2 intubations | Decannulation |
| 12 | 58 | F | N/A | None | No | No |
| 13 | 37 | F | 44 | diabetic neuropathy | No | No |
| 14 | 30 | F | 47 | Hypopituitarism, Cushingoid | No | No |
| 15 | 53 | F | 61.8 | None | No | No |
| 16 | 71 | M | 48 | MND | 4 years | No |
| 17 | 52 | F | 68 | None | No | No |
| 18 | 64 | F | 75 | None | No | No |
| 19 | 66 | F | 58 | None | No | No |
| 20 | 61 | M | 49 | Post-Polio | No | No |
| 21 | 50 | F | 67 | None | No | No |
| 22 | 66 | F | 56.3 | None | No | No |
| 23 | 69 | M | 58.6 | None | No | No |
| 24 | 42 | M | 74 | None | No | No |
| 25 | 37 | F | 66.8 | None | No | No |
| 26 | 59 | M | 55 | Polyneuropathy | No | No |
CNVS — continous noninvasive ventilatory support; BMI — body mass index.
BiPAP to intubation—“No” denotes patients who were placed on NVS settings from outset, otherwise patients developed acute on chronic respiratory failure with or without using bi-lovel PAP with seven requiring intubation then extubation to CNVS and five of the seven failing a total of nine extubation attempts at other hospitals before transfer for successfully extubation to CNVS and mechanical insufflation-exsufflation (MIE). One local patient required extubation to CNVS and MIE twice.
Required extubation/decannulation to CNVS – except where noted, all intubations were for acute on chronic respiratory failure and extubation attempts to CPAP, bi-level PAP, and O2 failed or were not attempted do to inability to pass ventilator weaning parameters and spontaneous breathing trials. The patients had to be extubated to CNVS and MIE.
Denotes patients who had been successfully extubated to low span bi-level PAP and O2 but remained extremely hypercapnic despite sleep bi-level PAP until being transitioned to NVS settings, in 5 cases, after being intubated again, undergoing tracheotomies, then being decannulated to CNVS in our center. Patient 9, however, only required sleep NVS post-decannulation.
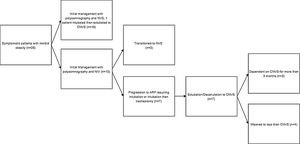
Patients’ initial presentation, clinical management, and transition to NVS are described in a flow diagram in Fig. 1. Diurnal CO2 and SpO2 levels, mean sleep SpO2 levels, and AHI for those undergoing polysomnograms before introduction to NVS are noted in Table 2. Extent of NVS dependence (VFBA) between NVS to CNVS varied with patients’ weight (Table 2).
Pre-Noninvasive Ventilatory Support (NVS) Assessment.
| Case | Pre-NVS | |||
|---|---|---|---|---|
| Daytime O2 Sat | Daytime EtCO2/TcCO2 | Sleep mean O2 Sat | AHI | |
| 1 | 88−94% | 51 | ||
| 2 | 92% | 71 | 81 | |
| 3 | 88−94% | 63 | ||
| 4 | 75−90% | 90% | ||
| 5 | 79−83 | |||
| 6 | 85−91% | 59 | 93% | |
| **7 | ** | |||
| 8 | 96−98% | 47 | ||
| 9 | 94−95% | 44 | ||
| 10 | 83−85% | 54 | 89% | 67 |
| 11 | 86−88% | 51 | ||
| 12 | 79−89% | 56 | ||
| 13 | 98% | 33 | 92−93% | 81 |
| 14 | 90−92% | 52 | 90% | 79 |
| 15 | 83−84% | 65 | 74 | |
| 16 | 91−94% | 48 | 89% | 40 |
| 17 | 78−82% | 103 | 55.2% | |
| 18 | 87% | 64 | 81% | 113 |
| 19 | 91% | 58 | 87% | 68 |
| 20 | 86% | 68 | 83% | 32 |
| 21 | 90% | 50 | 86% | 79.5 |
| 22 | 94% | 49 | 88% | 56.2 |
| 23 | 96% | 60 | 87% | 50.8 |
| 24 | 89% | 55 | 77% | 19.3 |
| 25 | 92% | 49 | 83% | 88.7 |
| 26 | 90−94% | 94 | 84% | 105 |
EtCO2 — End-tidal carbon dioxide in mm Hg; TcCO2 — transcutaneous CO2 mm Hg; Daytime O2 sat and EtCO2 — O2 sat and EtCO2 while stable; AHI — apnea hypopnea index; NVS settings — ** indicates pre-hospitalization blood gases unknown; the patient was transferred for extubation to CNVS after failing two extubation attempts and passing no ventilator weaning parameters; post-extubation was discharged using sleep NVS.
Table 3 denotes extent of daytime use of NVS. Five intubated patients were transferred, including two on two occasions, from other critical care units after failing weaning and a total of 15 extubation attempts. Two intubated patients were local. All seven unweanable patients were successfully extubated to CNVS and MIE, including Cases 3 and 8 on two occasions, and discharged home. Three of the seven eventually weaned, at least temporarily, to nighttime-only NVS. Their CPF averaged 208L/m (70L/m Case 8) and only one patient had flows over 270L/m (Case 15) so 15 needed access to MIE long-term as well as for extubation and/or decannulations.15–17
Duration of Noninvasive Ventilatory Support (NVS) Use and Post-NVS Parameters While Using NVS at the Noted Settings.
| Case | NVS extent and duration | NVS outcomes | ||||||
|---|---|---|---|---|---|---|---|---|
| Sleep only NVS | Night NVS+Day | CNVS | O2 Sat | EtCO2/TcCO2 | AHI with NVS | Day and Sleep NVS PIP (cmH20) | Sleep Bi-level Pressure mm Hg | |
| a1 | None | 5 years | 5 year | 95−100% | 39 | 48−52a | ||
| a2 | None | None | 1 year | 95–100% | 40 | 3 | 48−55a | |
| 3 | None | None | 1 year | 95−100% | 39 | 0.9 | 36−45 | 42/4 |
| a4 | None | None | 65 years | 95−100% | 41 | 35−40a | ||
| a5 | None | 1 year | 1 year | 91−92% | 40 | 40−45a | ||
| a6 | 8 years | None | Only for Ext/Dec | 95−97% | 42 | 25−28a | ||
| a7 | None | None | Only for Ext/Dec | 97−98% | 32 | 25−32a | ||
| a8 | 4 years | None | None | 96−98% | 47 | 32−38a | ||
| a9 | None | None | 1 month | 95−97% | 42 | 25−30a | ||
| 10 | 9 years | None | 2 years | 93−95% | 35 | 0.9 | 26−30 | 22/3 |
| 11 | 3.6 years | 6.4 years | None | 35−44 | 30/4 | |||
| a12 | None | 2.4 years | None | 94−95% | 44 | 32−36a | ||
| 13 | 6 months | None | None | 98% | 33 | 0.8 | 26−32 | 18−25/2 |
| 14 | None | 9 years | None | 98−99% | 39 | 2.3 | 25 | 20/2 |
| 15 | None | 2.5 years | 3 months | 94−95% | 44 | 2.1 | 48−55a | |
| a16 | 2 years | 1 year | 9 months | 95−100% | 37 | 3.1 | 34−39a | |
| a17 | None | None | 3 months | 95−100% | 35 | 48−55a | ||
| 18 | None | 5 years | 4 years | 97% | 33 | 2 | 40 | 35/4 |
| 19 | None | 4 years | 4 years | 97% | 42 | 3.8 | 30 | 24/4 |
| 20 | None | 12years | None | 98% | 35 | 1.3 | 60 | 35/4 |
| 21 | 2 years | None | 5 years | 95% | 42 | 4.4 | 30 | 25/5 |
| 22 | 6 years | None | None | 96% | 38 | 1.8 | 0 | 24/4 |
| 23 | 3 years | 3 years | None | 98% | 40 | 3.5 | 34 | 28/4 |
| 24 | None | None | 2 years | 96% | 36 | 1.1 | 38 | 28/4 |
| 25 | None | 6 years | 6 | 97% | 39 | 4.8 | 30 | 23/4 |
| 26 | 1 year | None | None | 98% | 38 | 0 | 30/4 | |
(C)NVS — (continuous) noninvasive ventilatory support; EtCO2 — end-tidal carbon dioxide in mm Hg; TcCO2 — transcutaneous CO2 mm Hg; NVS extent and duration — the total duration of use of sleep-only NVS, NVS use up to 23h a day, and CNVS with little or no ventilator free breathing ability, however, patients often varied from one category to another as their weights and vital capacities increased or decreased.
All six patients who presented using up to continuous TMV (CTMV) were successfully decannulated to NVS/CNVS and MIE including 3 CTMV users who had no VFBA. All six subsequently weaned to have at least some daytime VFBA. Two patients who were decannulated to CNVS but then used sleep-only NVS, subsequently developed pneumonia and required re-intubation but were successfully re-extubated to CNVS (Table 1: Cases 1, 5). One patient who was extubated to CNVS developed sepsis from a hand infection, underwent tracheotomy, and was subsequently decannulated to CNVS.
Dyspnea was relieved and blood gases normalized for all patients. For the eleven who used only sleep NVS the initial daytime EtCO2 of 61.5±6.3 decreased to 39.5±3.3mm Hg, and SpO2 normalized before increasing weight resulted in their need to extend NVS into daytime hours. The EtCO2 and SpO2 were always normal while using NVS during the day at PIPs of 25–55cm H2O. The mean pre-NVS AHI of 69.0±24.9 decreased to 2.3±1.4 during sleep NVS. Four required CNVS only for days to months following extubation or decannulation before weaning to less than full-time NVS was achieved. Using oximetry as feedback, all patients were able to maintain normal daytime SpO2 in ambient air by using NVS and MIE.17
For Center A, the 17 patients’ mean VC was 1409±871 (range 200–2680)ml when sitting, and 1029±764 (range 200–2060)ml when supine with a mean decrease of 27% from sitting to supine for these patients who could not tolerate reclining supine without using NVS. In the overall group, 18 patients used nasal and eight patients used oronasal interfaces for sleep NVS (Table 2). Four patients died; three predominantly from diabetes, hypertension, and renal failure including one with sickle cell anemia and aortic stenosis. Contact was lost with Case 8 who had liver cirrhosis and severe diabetes mellitus, and with case 13.
No patients were intubated due to failure of CNVS, however, two using less than CNVS, because of its inconvenience while walking, developed CO2 narcosis, required hospitalization and intubation for ARF, and had to be extubated back to CNVS.
Fourteen patients were CNVS dependent for 7.3±16.1 (range from 3 months to 66 years) years with little to no VFBA for a total of 92.2 patient-years. Six others predominantly used NVS day and night with some VFBA and the others predominantly for sleep-only (Table 2).
DiscussionThese results demonstrate that: (1) volume or pressure preset NVS settings can normalize blood gases day and night and AHIs without EPAP or PEEP; (2) ventilator unweanable patients with morbid obesity can be extubated/decannulated to, and depend on, CNVS for years to maintain normal blood gases without supplemental O2 despite having little or no autonomous ability to breathe, (3) CNVS can be safely provided day and night to PIPs over 40 and even over 50cm H2O for the morbidly obese, (4) morbidly obese patients are good candidates for mouthpiece and nasal CNVS because of intact bulbar-innervated musculature that permits them to comfortably use NVS settings, (5) and morbidly obese patients with respiratory orthopnea can use NVS for sleep reclining.
In many studies on managing morbid obesity, supplemental O2 and bi-level PAP at less than NVS settings are used rather than correcting SpO2 and CO2 levels by using NVS settings.18–24 Residual hypercapnia can be symptomatic and cause morbidity.9,25 Outside of academic circles, CO2 levels are often not routinely monitored during polysomnography. None of the patients we switched to active ventilator circuits with 0ml of PEEP had CO2 monitored during their sleep studies. While morbidly obese patients can obstruct inspiration during sleep and they may not exhale to atmospheric pressures, the air delivered at PIPs of 30–50cm H2O was unobstructed on flow signals from the ventilator download. Increasing the IPAP to compensate for the EPAP to achieve the same support as with EPAP increases mean thoracic pressures and possibly discomfort sometimes without obvious clinical benefit. Although the airway obstruction of patients with bulbar amyotrophic lateral sclerosis (ALS) is certainly pathologically distinct from that of the morbidly obese, Crescimanno et al. titrated the AHIs of patients with ALS then repeated the studies with 0 EPAP and reported that even 4cm H2O produced more leak than no EPAP, along with poorer sleep quality, more arousals, and a higher occurrence of patient-ventilator dyssynchrony without improving oxygen saturation.26 Thus, EPAP may also be unnecessary to treat the morbidly obese when NVS settings are used, since airflow was unobstructed, blood gases normalized, and symptoms relieved. Future studies will be needed to confirm this observation.
Pressure preset CNVS at 18–50cm H2O and volume preset CNVS at 700–1800ml have now been used to sustain life for over 65 years for some of the 257 post-poliomyelitis mouthpiece CNVS users described in 1993,27 for up to 28 years for 59 high level traumatic tetraplegics,28 for over 30 years for patients with Duchenne muscular dystrophy,15 for over 25 years for severe spinal muscular atrophy (SMA) type 129,33 for up to 13 years for ALS,30 as well as for others. None received EPAP, PEEP, or supplemental O2. Likewise, many morbidly obese patients with no VFBA and immediate apnea when unaided, may also be managed by NVS without EPAP or PEEP.
Six patients were decannulated despite dependence on up to CTMV with limited VFBA. Although these patients could not be weaned from ventilatory support, this is not unprecedented. Perhaps the first decannulated CTMV dependent patient was a 17year old high level spinal cord injured patient with 420ml of VC seated but 0ml supine. He had had a C1 fracture in March of 1967. He was decannulated to CNVS using a mouthpiece during the day. The ostomy closed in April 1969. He was euthanized after 38 years of CNVS in 2006. Another 91 TMV dependent patients, including two with 0ml of VC, were subsequently decannulated to CNVS in reports in 1988 and 1990.27,28,31 Seven remained CNVS dependent for 12.4±6.3 (range 1–22) years. Another 61 decannulated patients ventilator unweanable patients were reported in 2014,16 and in another report, Ceriana et al. decannulated 46 patients with at least 8h of VFBA to bi-level NIV in 2019.32
No baro or volutrauma was observed in our patients nor in many hundreds of patients with ventilatory pump failure dependent on CNVS for decades, many of whom, as with some of these patients, have performed LVR to pressures of 60–90cm H2O three times daily for decades.6,7 Thus, statements like “when ‘NIV’ becomes ineffective or is needed all day tracheotomy is necessary” need to be re-thought.33,34 Although the use of long-term CNVS and extubation to it along with MIE instead of TMV has now expanded to over 30 centers around the world,3 this is its first description for the morbidly obese. Other than this report there are as yet no other reports of continuous ventilatory support via noninvasive interfaces for any morbidly obese patients with little to no VFBA, and none have been reported to use MIE to increase cough flows to expel airway debris.18–24 However, eight of the 17 patients, for whom it was measured, had CPF less than 250L/m and, at times, as low as 130L/m.
Limitations of this study were that it was retrospective with data from 2 different centers (not always the same data points available), not all patients underwent polysomnography which could have been used to determine the effect on blood gases and sleep of adding EPAP/PEEP to the high inspiratory pressures that normalized CO2, the accuracy of ventilator software is speculative, and forms of bariatric surgery were not documented. Polysomnograms offer information about sleep architecture and arousals and can be instructive even when CO2 is not monitored. However, since blood gases were normalized and symptoms of dyspnea and somnolence relieved, it is clear that morbidly obese patients can become continuously ventilator dependent using noninvasive interfaces rather than tracheostomy tubes or supplemental O2 provided that oximetry is used as feedback. Leaks at any pressures or volumes are controlled largely by ventilatory drive with sedative medications and O2 avoided.10
In this paper we have described the use of NVS with high PIP or tidal volumes for patients with morbid obesity who continued to have daytime hypercapnia and symptoms despite nocturnal ventilatory support using bi-level ventilation. This strategy was also used for a group of morbidly obese patients who were unweanable from ventilator use. Using the NVS strategy, in some patients on a continuous basis, we were able to normalize daytime blood gases and relieve symptoms associated with respiratory insufficiency. No patient has required tracheostomy or oxygen therapy. Future studies need to compare the use of NVS to NIV in those with symptomatic hypercapnic morbid obesity to systematically evaluate the impact of this approach on health care resource use, quality of life, tolerance and survival. Continuous NVS is preferable to continuous TMV.35
Conflicts of interestFinancial disclosure statements have been obtained and the authors have no conflicts of interest to declare.
JB and MG have been treating and managing all the patients, contributed to the writing of the paper, and confirm the study objectives, procedures, and data are honestly disclosed. A.K. analyzed the data and contributed to the writing. T.P. is a respiratory therapist and PhD student who gathered and analyzed the data for the Center B subjects and contributed to the writing.