High altitude pulmonary hypertension (HAPH), a chronic altitude related illness, is associated with hypoxemia, dyspnea and reduced exercise performance. We evaluated ECG and pulse wave-derived markers of cardiovascular risk in highlanders with HAPH (HAPH+) in comparison to healthy highlanders (HH) and lowlanders (LL) and the effects of hyperoxia.
MethodsWe studied 34 HAPH+ and 54 HH at Aksay (3250m), and 34 LL at Bishkek (760m), Kyrgyzstan. Mean pulmonary artery pressure by echocardiography was mean±SD 34±3, 22±5, 16±4mmHg, respectively (p<0.05 all comparisons). During quiet rest, breathing room air or oxygen in randomized order, we measured heart-rate adjusted QT interval (QTc), an ECG-derived marker of increased cardiovascular mortality, and arterial stiffness index (SI), a marker of cardiovascular disease derived from pulse oximetry plethysmograms.
ResultsPulse oximetry in HAPH+, HH and LL was, mean±SD, 88±4, 92±2 and 95±2%, respectively (p<0.05 vs HAPH+, both comparisons). QTc in HAPH+, HH and LL was 422±24, 405±27, 400±28ms (p<0.05 HAPH+ vs. others); corresponding SI was 10.5±1.9, 8.4±2.6, 8.5±2.0m/s, heart rate was 75±8, 68±8, 70±10 bpm (p<0.05, corresponding comparisons HAPH+ vs. others). In regression analysis, HAPH+ was an independent predictor of increased QTc and SI when controlled for several confounders. Oxygen breathing increased SI in HH but not in HAPH+, and reduced QTc in all groups.
ConclusionsOur data suggest that HAPH+ but not HH may be at increased risk of cardiovascular mortality and morbidity compared to LL. The lack of a further increase of the elevated SI during hyperoxia in HAPH+ may indicate dysfunctional control of vascular tone and/or remodelling.
Living at high altitude, where hypobaric hypoxia enhances sympathetic tone, hypoxemia and progressively increases mean pulmonary artery pressure (mPAP), residents may be exposed to increased risk for sudden cardiac death (SCD)1 and cardiovascular disease (CVD).2 Cardiac adaption and maladaptation to hypobaric hypoxia have been described previously.3,4 Acute altitude exposure can prolong heart-rate corrected QT interval (QTc),5,6 a validated marker for SCD.7 Additionally, free reactive oxygen species are involved in the adaptive process to hypobaric hypoxia.8 They act as signaling molecules to transcribe hypoxic-inducible factor 1−α. Unfortunately, excessive reactive oxygen species concentration can cause vascular endothelial dysfunction, the major underlying factor promoting atherogenesis and CVD.9 Well acclimatized, healthy highlanders have elevated reactive oxygen species concentration without evidence of vascular dysfunction, which is in contrast to observations in highlanders with chronic mountain sickness (CMS).10,11 CMS is characterized by polycythemia, pulmonary hypertension and severe chronic hypoxemia.12 Apart from CMS, another chronic high altitude related illness is high altitude pulmonary hypertension (HAPH),12 which is associated with elevated mPAP and moderate chronic hypoxemia but without polycythemia. Highlanders with HAPH may also be at increased risk of CVD and SCD with potential consequences for morbidity and mortality compared to healthy highlanders and lowlanders.2,7,13
Therefore, our aim was to examine indices of risk of CVD and SCD in highlanders with and without HAPH at their altitude of residence. To elucidate the role of altitude, a lowlander control group was assessed near sea level. We hypothesized, that highlanders with HAPH would reveal markers for elevated risk of CVD and SCD compared to healthy highlanders and healthy lowlanders.
MethodsParticipantsHighlanders with HAPH (HAPH+) and HH, 21–75 y of age, both genders were recruited and studied at the Aksay health post (3250m, barometric pressure 515±1mmHg, room temperature 24.2±3.2°C), Kyrgyzstan. Participants had to be of Kyrgyz ethnicity, born, raised and currently living at >2500m. The diagnosis of HAPH was established by typical symptoms and a mPAP >30mmHg in the absence of excessive erythrocytosis (hemoglobin concentration in females <19g/dl, in males <21g/dl), and other present or diagnosed diseases that lead to hypoxemia (i.e., such as cardio-pulmonary diseases).12 Healthy lowlanders (LL), 21–75 y of age, both genders, of Kyrgyz ethnicity, born, raised and currently living in Bishkek (760m barometric pressure 692±2mmHg, room temperature 25.8±1.2°C), formed the lowlander control group. To eliminate the influence of smoking on the cardiovascular system, current heavy smokers (>10cigarettes per day), and subjects with a history of smoking >20pack-years were excluded from the study. Other exclusion criteria were diagnosed comorbidities or drug therapies that may have interfered with control of breathing, or pulmonary hypertension other than HAPH, in particular from left ventricular failure, lung disease or other conditions listed in the guidelines on pulmonary hypertension of the European Society of Cardiology.2 Data for the current study have been collected during the conduct of a previous study on HAPH, sleep apnea14 and cerebral oxygenation15 but the analysis of the ECG and pulse wave, the focus of the current study, have not been published. This study was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained, and the ethics committee of the National Center of Cardiology and Internal Medicine, Bishkek, Kyrgyzstan, approved the study (No. 01-7/219).
Design and interventionHighlanders traveled from their homes in the remote mountain areas by horse or car within one day to the study site at the Aksay health post. LL were studied in the National Center of Cardiology and Internal Medicine of Bishkek. The study design was according to a randomized single-blinded crossover protocol as detailed below. Participants rested in supine position breathing through a face-mask equipped with a reservoir bag and a one-way exhalation valve (non-rebreather oxygen mask, P.J. Dahlhausen & Co. GmbH, Cologne, Germany). Either ambient air (FiO2 0.21) delivered by a continuous positive airway pressure generator (REMstar, Philips Respironics, Zofingen, Switzerland), or oxygen (FiO2 1.0) at a flow rate of 10l/min from a pressurized bottle were breathed through the mask. After a baseline period of at least 10min, a 10min recording period took place with ambient air (or oxygen, according to randomization). After a 10min wash-out period with ambient air breathing (if FiO2 1.0 was used first), the procedure was repeated with the alternate gas. Patients were not allowed to sleep during the experiment. Blinding of participants was achieved by placing the breathing gas source out of their view.
MeasurementsA 4-lead electrocardiogram and the finger photoplethysmogram from pulse oximetry were continuously monitored with a sampling rate of 200Hz. Arterial oxygen saturation (SpO2) was assessed by finger pulse oximetry (Alice 5, Philips Respironics, Zofingen, Switzerland). QTc was calculated by the Bazett's formula.16 Stiffness index (SI) and wave D to wave A ratio (DA) of the second derivative of the finger plethysmogram, both predictors for CVD were assessed.17,18 SI was calculated as height of the participant divided by the time from the systolic to diastolic inflection point of the pulse waveform19 whereas DA was calculated as described by Takada et al.20 and Imanaga et al.21 A clinical examination, measurement of systemic blood pressure, echocardiography and arterial blood gas analyses were obtained as reported previously.14
StatisticsData are presented in median (quartiles). A per-protocol analysis was undertaken for the present study. Comparisons between groups were made with one-way ANOVA followed by post-hoc Scheffé multiple-comparisons or Kruskal–Wallis one-way analysis of variance followed by Mann–Whitney-U test as appropriate. Within group comparisons were made with dependent t-test or Wilcoxon signed ranks test as appropriate. ECG and pulse wave contour analysis was performed beat – by – beat over the last 2min of breathing FiO2 0.21 or 1.0, respectively. ECG/pulse wave analysis was performed with customized interactive software programmed in MatLab on mean waveforms ensemble-averaged by ECG triggering over 2min. The number of participants exceeding the critical threshold of QTc prolongation of >440ms was evaluated by the Pearson's Chi-squared test.5,7
In order to evaluate the effect of HAPH and residence at high altitude on QTc and SI independent of known confounders, such as age, gender, body mass index (BMI), mean arterial pressure and SpO2 under ambient air, univariable and multivariable regression analyses were employed. A significance level of p<0.05 was considered statistically significant.
ResultsOf 232 highlanders invited to the study, 105 refused to participate, 37 had to be excluded due to relevant comorbidities or other exclusion criteria. Ninety consented to participate and could be examined. Of 39 LL invited to the study, 5 had to be excluded due to comorbidities or other exclusion criteria. Thirty-four consented to participate and could be examined. Two participants were excluded from the final analysis because of poor quality of ECG recordings; data from a total of 122 participants were included in the analysis. Demographics are presented in Table 1. HAPH+ were slightly older and had a higher body weight than both control groups. In addition, HAPH+ were more hypoxemic and had a higher heart rate than HH and LL (Table 1).
Demographic characteristics of the participants.
| Healthy lowlanders | Healthy highlanders | Highlanders with HAPH | |
|---|---|---|---|
| N (% female) | 34 (35%) | 54 (39%) | 34 (47%) |
| Age, y | 40 (30;47) | 39 (32;48) | 52 (47;58)*¶ |
| BMI, kg/m2 | 26.6 (23.1;27.8) | 23.5 (21.3;25.6)* | 29.0 (24.5;31.8)*¶ |
| mPAP, mmHg | 15 (12;19) | 23 (19;26)* | 33 (31;36)*¶ |
| SpO2, % | 95 (94;97) | 92 (90;94)* | 89 (87;90)*¶ |
| Heart rate, bpm | 69 (62;75) | 69 (62;73) | 75 (70;80)*¶ |
| Systolic BP, mmHg | 117 (111;129) | 105 (97;111)* | 113 (103;127)¶ |
| Diastolic BP, mmHg | 76 (68;86) | 68 (61;74)* | 74 (65;84)¶ |
| Mean BP, mmHg | 89 (83;99) | 80 (73;86)* | 87 (78;99)¶ |
| Smoking, pack-years | 0 (0;2) | 0 (0;4) | 0 (0;5) |
Data presented as median (quartiles); HAPH: high altitude pulmonary hypertension; BMI: body mass index; mPAP: mean pulmonary artery pressure; SpO2: peripheral arterial oxygen saturation; BP: blood pressure.
*p<0.05 vs healthy lowlanders.
¶p<0.05 vs healthy highlanders.
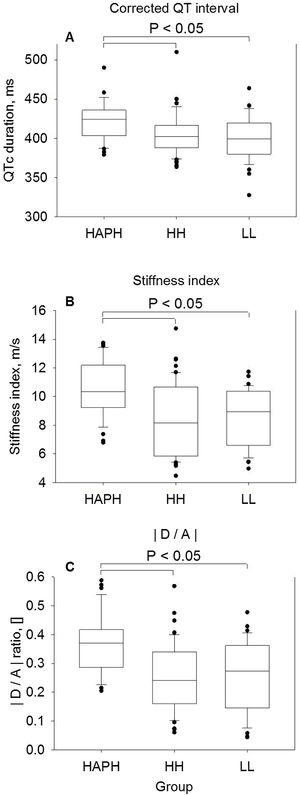
During breathing ambient air (Fig. 1, Panel A–C) QTc duration of HAPH+, HH and LL was, median (quartiles), 424 (404; 436)ms, 402 (388; 417)ms and 400 (380; 420)ms, respectively (p<0.05 vs HAPH+, all comparisons, p=NS between HH and LL). Correspondingly, SI of HAPH+, HH and LL were 10.4 (9.2; 12.2)m/s, 8.2 (5.9; 10.7)m/s and 9.0 (6.6; 10.4)m/s, respectively (p<0.05 vs HAPH+, all comparisons, p=NS between HH and LL); corresponding DA were 0.37 (0.29; 0.42), 0.24 (0.16; 0.34) and 0.27 (0.14; 0.36), respectively (p<0.05 vs HAPH+, all comparisons, p=NS between HH and LL). Critical QTc prolongation above 440ms were observed in 6 of 34 (18%) in HAPH+, 5 of 54 (9%) in HH and 3 of 34 (9%) in LL (p=NS all comparisons).
Comparison of baseline measurements under ambient air in highlanders with high altitude pulmonary hypertension (HAPH), healthy highlanders (HH) and healthy lowlanders (LL). Panel A: QT interval corrected for heart rate by the Bazett's formula.16 Panel B: Stiffness index, calculated by the height of the participant in meters divided by the time delay in seconds between the systolic and diastolic peaks (or, in the absence of a second peak, the point of inflection) of the pulse wave. Panel C: Wave D to wave A ratio of the second derivative of the finger plethysmogram. Boxes with lines represent medians and quartiles, whiskers represent the 10th and 90th percentiles, and dots represent individual values that fall outside the 10th–90th percentile range. Significant differences between groups are indicated. p<0.05 was considered significant.
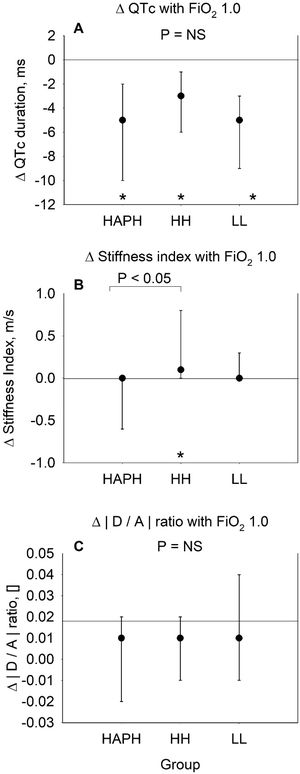
Breathing oxygen FiO2 1.0 (Fig. 2, Panel A–C) reduced QTc to a similar degree in HAPH+, HH and LL, i.e., by median difference (95% CI), −5 (−10 to −2)ms, −3 (−6 to −1)ms and −5 (−9 to −3)ms, respectively (p<0.05 compared to ambient air values within the different groups, p=NS between groups, Fig. 2, Panel A), so that HAPH+ remained at prolonged QTc duration compared to HH and LL. SI did not change with breathing oxygen in HAPH+, 0.0 (−0.6 to 0.0), p=0.585, and in LL, 0.0 (0.0 to 0.3), p=0.223, but SI slightly increased in HH with breathing oxygen, 0.1 (0.0 to 0.8), p<0.05, as shown in Fig. 2, Panel B. Ratio in DA remained stable in all 3 groups (Fig. 2, Panel C).
Changes with breathing oxygen (FiO2 1.0) in highlanders with high altitude pulmonary hypertension (HAPH), healthy highlanders (HH) and healthy lowlanders (LL) in comparison to breathing ambient air (FiO2 0.21). Panel A: Median change (95% confidence interval) in QT interval corrected for heart rate by the Bazett's formula.16 Panel B: Median change (95% confidence interval) in stiffness index, calculated by the height of the participant in meters divided by the time delay in seconds between the systolic and diastolic peaks (or, in the absence of a second peak, the point of inflection) of the pulse wave. Panel C: Median change (95% confidence interval) in wave D to wave A ratio of the second derivative of the finger plethysmogram. Asterisks (*) indicate significant changes (p<0.05) from baseline of the corresponding group. Significant differences between groups are indicated with horizontal lines. p<0.05 was considered significant.
Regression analysis confirmed that HAPH+ was an independent predictor of increased QTc and SI even when controlled for age, gender, mean arterial blood pressure, BMI, and SpO2 (Table 2).
Regression analysis of corrected QT interval (QTc) and stiffness index (SI) with several predictors.
| Univariable regression | Multivariable regression | |||||||
|---|---|---|---|---|---|---|---|---|
| Coeff | SE | 95% CI | p value | Coeff | SE | 95% CI | p value | |
| Dependent QTc, ms | ||||||||
| Age, y | 0.74 | 0.19 | 0.37;1.12 | <0.001 | 0.57 | 0.27 | 0.03;1.02 | 0.038 |
| Female vs. male | 15.07 | 4.57 | 6.01;24.12 | 0.001 | 12.06 | 4.70 | 2.75;21.38 | 0.012 |
| BMI, kg/m2 | 1.29 | 0.55 | 0.21;2.38 | 0.020 | 0.74 | 0.67 | −0.59;2.07 | 0.273 |
| SpO2, % | −1.45 | 0.69 | −2.82;0.08 | 0.038 | 0.79 | 0.78 | −0.76;2.35 | 0.313 |
| MAP, mmHg | −0.27 | 0.22 | −0.70;0.17 | 0.227 | −0.38 | 0.22 | −0.82;0.06 | 0.089 |
| Group membership | ||||||||
| HH vs LL | 5.91 | 6.02 | −6.02;17.84 | 0.329 | 5.83 | 6.54 | −7.14;18.79 | 0.375 |
| HAPH+ vs LL | 22.5 | 6.30 | 10.08;35.04 | <0.001 | 16.21 | 8.04 | 0.28;32.13 | 0.046 |
| Intercept | 300.4 | 84.2 | 133.6;467.2 | 0.001 | ||||
| Dependent SI, m/s | ||||||||
| Age, y | 0.11 | 0.02 | 0.08;0.14 | <0.001 | 0.12 | 0.02 | 0.08;0.16 | <0.001 |
| Female vs. Male | −0.11 | 0.44 | −0.98;0.76 | 0.805 | −0.18 | 0.39 | −0.96;0.60 | 0.650 |
| BMI, kg/m2 | 0.05 | 0.05 | −0.05;0.15 | 0.283 | −0.13 | 0.05 | −0.23;0.04 | 0.006 |
| SpO2, % | −0.13 | 0.04 | −0.20;−0.05 | 0.001 | 0.11 | 0.08 | −0.05;0.27 | 0.193 |
| MAP, mmHg | 0.05 | 0.02 | 0.01;0.09 | 0.012 | 0.03 | 0.02 | −0.01;0.07 | 0.163 |
| Group membership | ||||||||
| HH vs LL | −0.11 | 0.49 | −1.08;0.85 | 0.814 | 0.34 | 0.58 | −0.80;1.49 | 0.055 |
| HAPH+ vs LL | 2.04 | 0.47 | 1.11;2.97 | <0.001 | 1.71 | 0.66 | 0.40;3.02 | 0.011 |
| Intercept | −5.2 | 8.2 | −21.3;11.0 | 0.527 | ||||
All parameters from the univariate regression analyses were included in the multivariable models. BMI: body mass index; SpO2: arterial oxygen saturation; MAP: mean arterial pressure; HH: healthy highlanders; LL: healthy lowlanders; HAPH: highlanders with high altitude pulmonary hypertension.
All three indices of cardiovascular risk studied, the QTc a predictor of sudden cardiac death (SCD), the SI and the wave D to wave A ratio of the second derivative of the pulse plethysmogram, were exclusively increased in highlanders with high altitude pulmonary hypertension compared to healthy highlanders and lowlanders. Furthermore, HAPH+ had a higher heart rate compared to HH. Thus, the current study suggests a higher risk for SCD and CVD in HAPH+ compared to HH and LL. With 20min oxygen breathing (FiO2 1.0), QTc was reduced in HAPH+, however, QTc remained prolonged compared to HH and LL. The elevated values of the SI in HAPH+ along with the lack of an acute change during oxygen breathing may indicate an altered autonomic control of vascular tone and/or structural vascular remodeling. Taken together, our findings raise concerns that HAPH+ may be at increased risk of SCD and CVD.
These results are novel and of clinical importance since HAPH is common among highlanders with an estimated prevalence of 5–18%.22,23 With progressive increase of pulmonary artery pressure in untreated HAPH,24 QTc and SI may rise simultaneously, indicating that highlanders with long-standing, untreated HAPH may be at increased risk of latent CVD and SCD. In remote areas such as Aksay – plateau treatment for HAPH+ is difficult due to insufficient medication supply and lack of knowledge of the potential risks, which can result in premature death.
To the best of our knowledge, no previous studies have elucidated the risk for CVD and SCD in HAPH+, although several studies have shown an increased risk for SCD in highlanders with CMS7 and in travelers acutely exposed to high altitudes.25,26
Stiffness index and wave D to wave A ratioRimoldi et al. in 2012 and Bailey et al. in 2013, showed that highlanders with CMS had systemic vascular dysfunction, evidenced by impaired flow-mediated dilatation, increased vascular stiffness and increased carotid intima-media thickness, predisposing these patients to higher cardiovascular morbidity and mortality.10,11 A correlation between SpO2 and flow-mediated dilatation (r=0.62) and its improvement 1h after oxygen breathing in highlanders with CMS and in HH with moderate hypoxemia (SpO2<90%) led the authors to conclude that severity of hypoxemia was one of the underlying – and reversible – mechanisms responsible for the vascular dysfunction. However, they presented no results about mPAP and a possible correlation between mPAP and vascular dysfunction in CMS patients. In the current study we found high values of SI in HAPH+ comparable to those reported in patients with two or more cardiovascular risk factors,19 but SI were only weakly correlated with SpO2 (R2=0.045, p=0.001). The multivariable regression analysis revealed HAPH+, age and BMI as independent predictors of SI (Table 2). We found no impairments in the HH control group, which was in accordance with the cited study.11 It has been shown that chronic hypoxemia induces vasodilatation in the peripheral blood vessels,27 therefore, we assumed that baseline vessel diameter in the HAPH+ and HH would be larger compared to LL and would respond to breathing FiO2 1.0 by hyperoxia-induced vasoconstriction and elevation of SI in HAPH+ and HH compared to LL. Interestingly, breathing FiO2 1.0 increased SI in HH but not in HAPH+. This finding indicates that mildly hypoxemic HH have preserved vasomotor reactivity to breathing FiO2 1.0. Indeed, acute supplemental oxygen has shown to increase vascular stiffness and reactivity in healthy subjects and in diseases associated with mild hypoxemia, such as in COPD,28 indicating that breathing FiO2 1.0 modulates the vasomotor activity by enhancing parasympathetic activity and reducing sympathetic activity (indicated in our study by a hyperoxia-induced median reduction in heart rate of −6bpm (IQR, −8 to −5) and −7bpm (IQR, −9 to −5) in HH and HAPH+, respectively), or by reducing the bioavailability of nitric oxide due to increased oxidative stress.29 These changes towards higher vascular stiffness have been associated with enhanced spontaneous baroreflex sensitivity and restoration of vascular tone towards normal physiology in otherwise hypoxemic individuals.28 Although, heart rate decreased similarly under breathing FiO2 1.0 compared to room air in HH and HAPH+, the absence of hyperoxia-induced vascular stiffness as seen in HAPH+ might be due to only 20min hyperoxic gas breathing compared to 30min or 1h in the cited studies,11,29 or due to blunted vasoconstrictive mechanisms or even structural remodeling caused by more severe hypoxemia or pulmonary hypertension compared to HH. In LL, SI may possibly have remained unchanged due to the already well oxygenated blood under ambient air.
SI and wave D to wave A ratio of the second derivative of the finger plethysmogram, both predictors for CVD, correlated good to each other (R2=0.48, p<0.001).
In addition to the elevated mPAP, systemic blood pressure and heart rate were also elevated in HAPH+ compared to HH and LL. These findings are consistent with an elevated sympathetic tone.
Sudden cardiac deathQTc was prolonged in HAPH+ compared to HH and LL. Furthermore, QTc prolongation could be reduced with oxygen administration, but remained prolonged compared to the two control groups. Multivariable regression analysis revealed HAPH+, gender and age as independent factors influencing QTc. Our results are in accordance with past studies, showing an association between QTc and elevated pulmonary arterial pressure,30 gender31 and age.32 Right ventricular strain and the association with sleep apnea with cyclic nocturnal deoxygenation contribute to impaired repolarization of cardiac muscles and may therefore reflect an increased risk of ventricular tachycardia and fibrillation.
LimitationFor logistical reasons, the current study in the remote mountain region could only include a relatively small sample of highlanders compared to previous low altitude studies.33 Instead of a 12-lead ECG, we used a 4-lead ECG and were only able to analyze lead II ECG wave contour. SI assessed by digital volume pulse as a predictor for arterial stiffness is a common noninvasive technique to assess arterial stiffness and has been successfully validated against flow mediated dilatation34 or tonometry-derived augmentation index.19,34 HAPH+ were significantly older compared to the two control groups, however, in multivariable regression models controlling for age, gender and other confounders, HAPH+ remained an independent predictor for SCD and CVD.
ConclusionECG and pulse wave-derived indices studied in the current investigation in highlanders with high altitude pulmonary hypertension indicate that they may be at greater risk for cardiovascular diseases and sudden cardiac death compared to healthy highlanders and lowlanders in whom QTc and SI were normal. Breathing oxygen reduced QTc in all groups, however, this marker of sudden cardiac death remained elevated in HAPH+. Moreover, the elevated SI and A to D wave ratio derived from the finger pulse plethysmogram, both predictors of cardiovascular disease, remained unchanged in HAPH+ during hyperoxia consistent with altered vessel wall properties. In summary, our observations raise concerns that HAPH+ might be at increased risk of SCD and CVD.
FundingThis work was supported by the OPO Foundation and the Zurich-Lung-League.
Conflict of interestThe authors declare no conflict of interest.
The study was supported by grants from the OPO Foundation and the Lunge Zurich, Switzerland. Philips Respironics Switzerland contributed some of the measurement equipment used in this study. Siemens Healthcare Diagnostics AG, Zurich, Switzerland, provided the blood gas analyzer.