The World Health Organization (WHO) recommends countries introduce new anti-TB drugs in the treatment of multidrug-resistant tuberculosis.
The aim of the study is to prospectively evaluate the effectiveness of bedaquiline (and/or delamanid)- containing regimens in a large cohort of consecutive TB patients treated globally.
This observational, prospective study is based on data collected and provided by Global Tuberculosis Network (GTN) centres and analysed twice a year.
All consecutive patients (including children/adolescents) treated with bedaquiline and/or delamanid were enrolled, and managed according to WHO and national guidelines.
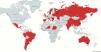
Overall, 52 centres from 29 countries/regions in all continents reported 883 patients as of January 31st 2021, 24/29 countries/regions providing data on 100% of their consecutive patients (10–80% in the remaining 5 countries).
The drug-resistance pattern of the patients was severe (>30% with extensively drug-resistant -TB; median number of resistant drugs 5 (3−7) in the overall cohort and 6 (4−8) among patients with a final outcome).
For the patients with a final outcome (477/883, 54.0%) the median (IQR) number of months of anti-TB treatment was 18 (13−23) (in days 553 (385–678)). The proportion of patients achieving sputum smear and culture conversion ranged from 93.4% and 92.8% respectively (whole cohort) to 89.3% and 88.8% respectively (patients with a final outcome), a median (IQR) time to sputum smear and culture conversion of 58 (30−90) days for the whole cohort and 60 (30−100) for patients with a final outcome and, respectively, of 55 (30−90) and 60 (30−90) days for culture conversion.
Of 383 patients treated with bedaquiline but not delamanid, 284 (74.2%) achieved treatment success, while 25 (6.5%) died, 11 (2.9%) failed and 63 (16.5%) were lost to follow-up.
The World Health Organization (WHO) estimated that about half million people suffer from multidrug- (MDR-) or rifampicin-resistant (RR-) tuberculosis (TB) in 2019, of whom 38% accessed treatment and, among them, 57% were successfully treated.1,2
Effective treatment, coupled with rapid and accurate diagnosis, of both drug-susceptible and -resistant (MDR- and extensively drug-resistant, XDR-) TB cases is an essential intervention to curb the TB epidemic and prevent further development and transmission of drug-resistant Mycobacterium tuberculosis strains.3,4
New drugs (i.e., delamanid and bedaquiline) have been recently licensed to manage MDR- and XDR-TB2; they were included into a new WHO drug classification where bedaquiline belongs to Group A and delamanid to Group C.5–18
Although more evidence is becoming available from experimental and observational studies on the efficacy and effectiveness of new drugs,19–23 programmatic information on their effectiveness is still incomplete worldwide.1
The Global Tuberculosis Network (GTN) project,24 which recently reported on the safety of these drugs, allowed to shed further light on the effectiveness of these drugs in a large cohort of patients (Table 1, Fig. 1).24–26
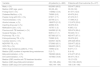
Participating countries, estimated coverage and number of cases enrolled.
| Countries | Estimated coveragea % | Cases enrolled N |
|---|---|---|
| Argentina | 100 | 11 |
| Australiac | 100e | 26 |
| Belarusb | 80 | 53 |
| Belgium | 60 | 3 |
| Brazil | 100 | 39 |
| Bulgaria | 100 | 17 |
| Chile | 100 | 1 |
| Chinac | 100d | 5 |
| Eswatini | 100 | 41 |
| Greece | 100 | 6 |
| India | 100e | 15 |
| Italyg | 80 | 40 |
| Latvia | 100 | 30 |
| Lithuaniah | 100 | 160 |
| Mexicoi | 100 | 11 |
| Nepalh | 100 | 125 |
| Netherlandsb | 100 | 6 |
| Niger | 100 | 17 |
| Paraguay | 100 | 1 |
| Peru | 80 | 29 |
| Portugal | 100 | 1 |
| Romaniac | 100f | 7 |
| Russian Federationb | 100j | 202 |
| Slovakia | 100 | 1 |
| Spaing | 100 | 8 |
| Sudan | 100 | 2 |
| Sweden | 100 | 19 |
| Switzerland | 100d | 3 |
| United Kingdom | 10 | 4 |
| Total 29 | Range 10%–100% | Total 883 |
Legend:
The aim of the study is to prospectively evaluate the effectiveness of bedaquiline and/or delamanid- containing regimens in a cohort of consecutive TB patients treated globally.
MethodsStudy designThe study is observational, prospective and based on the collection twice a year and analysis of data provided by GTN centres.
Following a pilot study implemented in 2015 to pre-test the project’s feasibility, the results of the project (management of adverse events) was published elsewhere.25,26
The study was approved by the Ethics Committee of the coordinating centre, and the participating centres obtained ethical clearance based on local regulations and signed a data-sharing agreement.25,26
All consecutive patients (including children and adolescents) treated with bedaquiline and/or delamanid were enrolled either from the beginning of the study or from the time the drugs under study were introduced in the respective country centre (e.g. in Mexico, Nepal, Paraguay, Spain, Slovakia and Sudan).25,26
In all participating countries, the patients were managed according to WHO and National guidelines, under supervision of a coordinating team supervising the patients’ clinical management and validation of data.27 Investigators were contacted by the coordinating centre to ensure accuracy after recoding and validation of the dataset before final analysis was conducted. Discrepancies were resolved by consensus.
WHO case and treatment outcome definitions were used.1,5,6,28,29
The present manuscript reports the results of the interim analysis conducted on the data collected up to the 31st January 2021.
Variables collectedThe data were collected via an ad hoc developed collection form in electronic format.25,26
The information collected (from the clinical files of the participating centres) included, among others, anonymized patient’s demographic data, bacteriological, radiological and clinical status at diagnosis, and details on bacteriological conversion and final treatment outcomes.
The study coverage and number of patients treated per centre are reported in Table 1.
Data analysisA descriptive analysis was performed to evaluate the characteristics of the cohort.
Qualitative and quantitative variables were summarised using absolute and relative (percentage) frequencies, medians with interquartile ranges (IQR), and means with standard deviations (SD). Sputum smear and culture conversion (as well as the time to sputum and culture conversion) were evaluated in the whole cohort and in those completing their prescribed regimen.
Treatment outcomes were evaluated only in patients who completed the prescribed treatment regimen (separately for the entire cohort and in patients treated with bedaquiline but not delamanid) to favour international comparisons.
ResultsOverall, 52 centres from 29 countries/regions in all continents reported 883 patients as of January 31st 2021 (Fig. 1).
Argentina, Australia (State of Victoria), Brazil, Bulgaria, Chile, Eswatini, China (Zhejiang Province), Greece, India, Latvia, Lithuania, Mexico, Nepal, The Netherlands, Niger, Paraguay, Portugal, Romania, Russian Federation (Moscow and Arkhangelsk Oblasts), Slovakia, Spain, Sudan, Sweden and Switzerland (Vaud county) reported 100% of the patients treated with new drugs in the country/region, while Belarus, Belgium, Italy, Peru and the United Kingdom reported a proportion of national patients ranging from 10% to 80% (Table 1).
Demographic, epidemiological, and clinical characteristics of the patients are summarised in Table 2. The bacteriological conversion rates and the time to sputum smear and culture conversion are reported in Table 3 separately for the entire cohort and for the patients completing their prescribed treatment regimen. The final treatment outcomes of the entire cohort (477/883, 54.0%) are summarized in Table 4, while Table 5 is reporting the patients treated with bedaquiline only who have a final outcome assigned.
Characteristics of 883 patients undergoing treatment with bedaquiline and delamanid in the cohort, including 477 who completed the prescribed regimen.
| Variable | All patients (n = 883) | Patients with final outcome (N = 477) |
|---|---|---|
| Male, n (%) | 602/883 (68.2) | 333/477 (69.8) |
| Median (IQR) age, years | 38 (28−49) | 39 (30−50) |
| Foreign born n (%) | 118/882 (13.4) | 61/477 (12.8) |
| Diabetes Mellitus, n (%) | 79/880 (9.0) | 40/476 (8.4) |
| People living with HIV, n (%) | 67/871 (7.7) | 27/473 (5.7) |
| Thyroid disease, n (%) | 25/795 (3.1) | 17/399 (4.3) |
| Alcohol misuse, n (%) | 186/879 (21.2) | 112/475 (23.6) |
| Injecting drug user n (%) | 48/880 (5.5) | 30/475 (6.3) |
| Methadone user, n (%) | 10/787 (1.3) | 4/395 (1.0) |
| Previous anti-TB treatment, n (%) | 544/880 (61.8) | 329/474 (69.4) |
| Surgical therapy, n (%) | 90/814 (11.1) | 59/449 (13.1) |
| Pulmonary TB, n (%) | 857/883 (97.1) | 463/477 (97.1) |
| Extra-pulmonary TB, n (%) | 72/882 (8.2) | 39/476 (8.2) |
| Cavitary lesions, n (%) | 523/831 (62.9) | 295/448 (65.8) |
| MDR/RR-TB, n (%) | 575/883 (65.1) | 300/477 (62.9) |
| XDR-TB, n (%) | 289/883 (32.7) | 169/477 (35.4) |
| Other drug-resistance patterns, n*(%) | 19/883 (2.2) | 8/477 (1.7) |
| Median (IQR) number of reported drug-resistances | 5 (3−7) | 6 (4−8) |
| Bdq administration, n (%) | 782/883 (88.6) | 416/477 (87.2) |
| Dlm administration, n (%) | 167/883 (18.9) | 94/477 (19.7) |
| Median (IQR) months anti-TB treatment duration | – | 18 (13−23) |
| Median (IQR) days Bdq administration | 180 (168−264) | 183 (168−363,5) |
| Median (IQR) days Dlm administration | 168 (144−184) | 168 (136−186) |
TB: tuberculosis; IQR: interquartile range; Bdq: bedaquiline; Dlm: delamanid; MDR/RR-TB: multi-drug resistant /rifampicin-resistant tuberculosis; XDR-TB: extensively drug-resistant tuberculosis.
Sputum smear and culture conversion and median time to bacteriological conversion in 883 patients treated with new anti-tuberculosis drugs.
| Variable | All patients (n = 883) | Patients with final outcome (N = 477) |
|---|---|---|
| Sputum smear conversion, n (%) | 467/500 (93.4) | 274/307 (89.3) |
| Sputum culture conversion, n (%) | 532/573 (92.8) | 324/365 (88.8) |
| Median (IQR) days sputum smear conversion | 58 (30−90) | 60 (30−100) |
| Median (IQR) days sputum culture conversion | 55 (30−90) | 60 (30−90) |
IQR: interquartile range.
Treatment outcomes of the 477 patients who completed the prescribed regimen including new anti-tuberculosis drugs.
| Treatment Outcome | n/N (%) |
|---|---|
| Treatment success (cured + treatment completed) | 344/477 (72.1) |
| Cured | 281/477 (58.9) |
| Treatment completed | 63/477 (13.2) |
| Died | 38/477 (8.0) |
| Failure | 20/477 (4.2) |
| Lost to follow-up | 75/477 (15.7) |
Treatment outcomes of the 383 patients treated with bedaquiline (but no delamanid) who completed the prescribed regimen.
| Treatment outcome | n/N (%) |
|---|---|
| Treatment success (cured + treatment completed) | 284/383 (74.2) |
| Cured | 226/383 (59.0) |
| Treatment completed | 58/383 (15.1) |
| Died | 25/383 (6.5) |
| Failure | 11/383 (2.9) |
| Lost to follow-up | 63/383 (16.5) |
Overall, 883 patients were treated with bedaquiline and/or delamanid, 477 of them with a final outcome assigned (Table 2).
Most patients were male (n = 602, 68.2% in the overall cohort and n = 333, 69.8% with a final outcome assigned) and the median (IQR) age was 38 (28–49) years for the entire cohort and (30–50) years for those with a final outcome.
The proportion of foreign born was 13.4% in the overall cohort and 12.8% in the group of patients with a final outcome. The main co-morbidities and risk factors are summarized in Table 2.
Pulmonary TB was diagnosed in 97% patients, and cavity lesions were found in over 60% of the patients’ radiographs. Overall, there were 575/883 (65%) patients with MDR/RR-TB in the overall cohort, of whom 300/477 (62.9%) with a final outcome. Among them the XDR-TB cases were, respectively 289/883 (32.7%) and 169/477 (35.4%). Other resistance patterns were present only in 19/883 (2.2%) and 8/477 (1.7%), respectively.
The median (IQR) number of drugs for which resistance was detected was 5 (3−7) in the overall cohort and 6 (4−8) among patients with a final outcome.
Bedaquiline was administered to 782/883 patients in the entire cohort (88.6%), of whom 416/477 (87.2%) with a final outcome. The patients undergoing treatment with delamanid were, respectively 167/883 (18.9%) and 94/477 (19.7%), some of them having been treated also with bedaquiline.
The median (IQR) number of months of anti-TB treatment was 18 (13−23) (in days 553 (385–678)) among patients with a final outcome.
Bedaquiline was prescribed for 180 (168−264) days in the whole cohort and 183 (168–364) among patients with a final outcome. Delamanid was prescribed for 168 (144−184) days in the entire cohort and 168 (136−186) days to patients with a final outcome.
The proportion of patients achieving sputum smear conversion was 93.4% in the whole cohort and 89.3% among the patients with a final outcome, with a median (IQR) time to sputum smear and culture conversion of 58 (30−90) days for the whole cohort and 60 (30−100) for patients with a final outcome and, respectively, of 55 (30−90) and 60 (30−90) days as far as culture conversion is concerned (Table 3).
The final treatment outcomes of the entire cohort (477/883, 54.0%) are summarized in Table 4; 344/477 patients (72.1%) achieved treatment success, 38 died (8%), 20 failed (4.2%) and 75 (15.7%) were lost to follow-up.
Among the 383 patients treated with bedaquiline but not delamanid, 284 (74.2%) achieved treatment success, while 25 (6.5%) died, 11 (2.9%) failed and 63 (16.5%) were lost to follow-up (Table 5).
DiscussionThe aim of the present study was to prospectively evaluate the outcome of a global cohort of patients treated with new anti-TB drugs.
Although new research results (some summarized in a special bedaquiline series of the IJTLD)19–23,30–35 have been recently published, to the best of our knowledge this is the first global study prospectively reporting detailed information on treatment outcomes; the report of the safety profile of the new drugs was published elsewhere.25,26
The results of our study show that ∼90% of patients from 29 countries in all continents, with a severe pattern of drug resistance (>30% with XDR-TB; median number of resistances: 5–6) achieved sputum smear and culture conversion within 60 days of treatment with new anti-TB drugs. The success rates achieved were 72.1% in the full cohort (patients with a final outcome) and 74.2% among the patients (the vast majority) treated with bedaquiline. This second outcome is particularly relevant for international comparisons. Importantly, in this specific group of patients the death rate was 6.5%, the failure rate 2.9% and the lost to follow-up rate 16.5%; these outcomes need to be read considering that this cohort has been programmatically managed in many different settings, with a relatively low prevalence of HIV infection (5.7%). In a previous study by the GTN with different patients treated with bedaquiline,9 the culture conversion rate was similar (90%) and the overall success rate 76.9%
The study stratified the success rates by geographical area, showing that in austral Africa (where the HIV prevalence is higher) it was lower (64.6%) than in Niger (76.5%) where HIV prevalence is low, Europe (76.5%) and elsewhere (77.6%). Specifically in XDR-TB patients, the success rate was 77.6% in Africa, 80.4% in Europe and 77.7% elsewhere; these peculiar results, with treatment outcomes higher among XDR- than MDR-TB patients, have been caused by the fact that the XDR-TB patients had access to better drugs in the regimen (e.g. linezolid, clofazimine) which were not available in all countries (at the time the study was conducted) for MDR-TB patients. The WHO has from January 2021 updated its DR-TB definitions36 to include the term pre-XDR for patients with an MDR-TB strain resistant to later generation fluoroquinolone and XDR-TB which is MDR-TB plus resistance to two group A drugs (fluoroquinolone plus bedaquiline or linezolid resistance). The MDR-TB definition remains the same.28,36
Similarly, the death rate was much higher in Africa (23.9%) than in Europe (3.5%) and elsewhere (6.1%).
In a sub-group analysis of the 57 severe patients undergoing adjuvant surgery, the culture conversion rate was similar (90%) and the overall success rate 69.1%.37
A South African study on 19,617 patients showed a 3-fold reduction of all-cause mortality among individuals treated with bedaquiline when compared with those treated without new drugs.38
In the large individual patient data meta-analysis on 12,030 MDR-TB patients18 a small proportion of patients was treated with bedaquiline: 431/491 (87.8%) achieved treatment success (aOR, 95% CI: 2.0, 1.4–2.9) and 59/550 (10.7%) died (aOR, 95% CI: 0.4, 0.3−0.5).
The preliminary results of the Challenge-TB Project30 reported, among bedaquiline-treated patients, a culture conversion of 71% at months 6, with 58.8% treatment success, 11.8% failure, 23.5% died, 4.7% lost to follow-up and 1.2% still on treatment after 24 months (2016 cohort data). The patients’ drug resistance profile was not reported.30 The project involved 23 countries with 9389 patients enrolled between 2016 and mid-2019. Among the most relevant problems encountered, the Authors identified the limited in-country coordination and the absence of a robust clinical and laboratory network and the difficulties of implementing effective monitoring of adverse events related to the new drugs.25,26
A report from India (interim analysis) showed 83% culture conversion rate among the patients treated with bedaquiline within a median time of 60 days, while the final outcomes were not yet available.33
In Eswatini, between 2015 and 2018, 355 patients started treatment with new drugs (bedaquiline and/or delamanid), of whom 109 were treated with bedaquiline only and with final outcomes.35 Out of 109 patients, 80 were treated successfully (73.4%, a result consistent with that of our study), 18 died (16.5%, higher than our death rate but the HIV prevalence was higher in Eswatini, 72.3%), 1 failed, 1 was lost to follow-up (both 0.9%) and 9 were still in treatment after 24 months (8.3%). In the Eswatini cohort the proportion of males (58−7%), the median (IQR) age (35 (29–44) years) and the proportion of pre-XDR-/XDR-TB patients (26−1%) was consistent with our study.
Although without reporting details on treatment outcomes some studies have started reporting on the modified shorter regimens, which include bedaquiline ro replace the injectable drug in the former Bangladesh regimen.20,31,34
The project worked as a ‘register’ reporting treatment outcomes (and aDSM findings)25,26 twice a year so as to support countries in implementing quality monitoring and evaluation of the process of introducing new anti-TB drugs under programmatic conditions.
The study has several strengths, including the number of countries participating (29) from all continents, a large sample size (as far we know one of the largest studies of its kind), the prospective design, and the accuracy of the information collected. Last but not least, the majority of countries/states/regions (24/29) provided data on all the consecutive patients treated with bedaquiline and delamanid during the study period.
One limitation (common to all the studies of this kind) is the impossibility of attributing the outcomes to a specific drug, as treatment regimens are inherently polypharmacological.
A second limitation is that few paediatric patients (twenty-seven individuals aged less than 18 years) and people living with HIV (n = 67; 7.7%) were included in the cohort to allow specific sub-analyses.
The study will continue to evaluate early and final treatment outcomes as periodic updates occur and the ‘cohort’ is therefore a ‘living’ one. This cohort allows evaluation of novel treatments and combinations in a relatively short time-frame – particularly important given the substantial variation in international practice and guidelines recommending person-centered therapy for MDR-TB.4,5,39–41
This approach will allow the participating countries to evaluate the ‘quality’ of their treatment services and minimise the risk of post-treatment sequelae responsible of functional damage and impaired quality of life.42–48
In conclusion, our Global TB Network study further supports the importance of access to lifesaving anti-TB drugs like bedaquiline to improve outcome of drug-resistant (DR) TB patients. Bedaquiline has allowed for an all-oral, less toxic shorter regimen which significantly improves survival, and it is becoming more widely available globally.49 Future cohort reviews will show a reduction of treatment duration from 18 months to 6 months. This global study shows that even when there is access to the same WHO DR TB regimen, outcomes can still differ greatly, highlighting that managing MDR-/DR-TB is not only a question of better detection of DR-TB and starting treatment. Even though the WHO has shortened treatment considerably for the majority of DR-TB patients, it is very likely that more work and investment are required, especially in resource limited settings and treatment of people living with HIV and to combat the small but concerning number of XDR-TB patients.
Authors’contributionThe manuscript was conceived, planned, written, edited and approved using a collaborative approach, following the internal GTN (Global Tuberculosis Network) and internationally acknowledged rules on Authorship, based on major intellectual contribution to the steps mentioned above. The study represents a global effort involving 26 countries in all continents.
Giovanni Sotgiu, Simon Tiberi, Rosella Centis, Lia D'Ambrosio and Giovanni Battista Migliori wrote the protocol. Giovanni Sotgiu, Laura Saderi and Raquel Duarte revised it for the methodological content.
Giovanni Sotgiu, Laura Saderi, Rosella Centis and Lia D’Ambrosio performed the analysis.
Simon Tiberi, Rosella Centis, Lia D'Ambrosio, Emanuele Pontali, Jan-Willem Alffenaar, Jose A. Caminero, Giovanni Sotgiu and Giovanni Battista Migliori wrote the first draft of the manuscript.
Sushil Koirala, Sergey Borisov, Judith Bruchfeld, Alberto Piubello, Onno Akkermann, Justin Denholm, José-María García-García, Rafael Laniado-Laborín, Jesica Mazza-Stalder, Alberto Matteelli, Marcela Munoz-Torrico, Martin van den Boom, Dina Visca, Jose A. Caminero, Giovanni Sotgiu wrote the sections of the manuscript (second draft).
Antonio Spanevello, José-María, García-García Zarir Farokh Udwadia, Edvardas Danila, Andrei Maryandyshev, Susanna Esposito and Margareth Dalcolmo provided comments to the second draft (third draft).
Bhabana Shrestha, Satya Raj Shakya, Hikmat Bahadur Khadka, Ghan Shyam Koirala, Rajesh GC, Skaidrius Miliauskas, Liga Kuksa, Selene Manga, Alena Skrahina, Saulius Diktanas, Luigi Ruffo Codecasa, Alena Aleksa, Antoniya Koleva, Evgeny Belilovski, Enrique Bernal, Martin J Boeree, Julen Cadiñanos Loidi, Qingshan Cai, Jose Joaquín Cebrian Gallardo, Moschos Charalampos, Edita Davidavičienė, Lina Davies Forsman, Jorge De Los Rios Jefe, Alemayehu Duga, Seifeldin Eltaeb Elamin, Nadia Escobar Salinas, Maurizio Ferrarese, Aleksey Filippov, Blagovesta Gadzheva, Ana Garcia, Ieva Gaudiesiute, Regina Gayoso, Roscio Gomez Rosso, Vygantas Gruslys, Gina Gualano, Wouter Hoefsloot, Victor Ionel Grecu, Jerker Jonsson, Elena Khimova, Heinke Kunst, Yang Li, Nomthandazo Lukhele, Cecile Magis-Escurra, Gugulethu Madonsela, Vinicio Manfrin, Valentina Marchese, Elena Martínez Robles, Andrei Maryandyshev, Marius Matei, Ilaria Motta, Hamdan Mustafa Hamdan, Birutė Nakčerienė, Lauren Nicod, Magnolia Nieto Marcos, Domingo Juan Palmero, Fabrizio Palmieri, Apostolos Papavasileiou, Marie-Christine Payen, Agostina Pontarelli, Sarai Quirós, Adrian Rendon, Laura Saderi, Agnese Šmite, Ivan Solovic, Mahamadou Bassirou Souleymane, Marina Tadolini, Marisa Vescovo, Piero Viggiani, Rolandas Zablockis, Dmitry Zhurkin and provided additions to the fourth draft.
Simon Tiberi and Justin Denholm proof read the manuscript.
All co-Authors approved the final manuscript.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approvalEthical approval was obtained by the coordinating centre and in each country as per national regulations in force.
Conflicts of interestThe authors have no conflicts of interest to declare.
The project is supported by the Global Tuberculosis Network (GTN; Committees on TB Treatment, Clinical trials and Global TB Consilium) and was part of the European Respiratory Society Latin American project in collaboration with ALAT (Asociación Latino Americana de Torax - Latino American Thoracic Association) and SBPT (Brazilian Society of Pulmonology and Tuberculosis).
This article belongs to the scientific activities of the WHO Collaborating Centre for Tuberculosis and Lung Diseases, Tradate, ITA-80, 2017-2020- GBM/RC/LDA.
The Authors wish to thank Dr. Algirdas Gauronskis, Dr.Vita Globytė (Clinic of Tuberculosis and Pulmonology, Republican Šiauliai county hospital, Šiauliai, Lithuania), Dr. Antanas Strazdas (Department of Tuberculosis, Alytus County Tuberculosis Hospital, Alytus, Lithuania), Dr. Paola Castellotti (Regional TB Reference Centre, Villa Marelli Institute, Niguarda Hospital, Milan, Italy), Ms. Samriddhi Karki (Kalimati Chest Hospital/ GENETUP NATA, Nepal), Dr Andrea Calcagno, Alberto Gaviraghi and Francesca Alladio (Department of Medical Science, Unit of Infectious Diseases, University of Torino, Italy) for their contribution.