The World Health Organization recommends the use of oral medications for the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB).1–3 However, few countries have adopted these recommendations, either due to lack of medication or because of outdated local recommendations, as in the case of Peru.4 The combination of some medications for the treatment of MDR TB such as delamanid (Dlm) with bedaquiline (Bdq) has been associated with a potential risk of cardiovascular symptoms such as prolongation of the QT interval corrected by Fridericia interval (QTcF).5 Other medications that may enhance the prolongation of this interval are fluroquinolones, especially moxifloxacin (Mfx) and clofazimine (Cfz). We present a case of the simultaneous use of these medications in a patient who provided informed consent. There was no prolongation of the QTcF interval, and no significant cardiac symptoms were observed throughout the entire treatment. (1) This patient was the first Peruvian to receive a completely oral combination of medications for the treatment of MDR tuberculosis.8 (2) This was the first Peruvian case to receive a combination of 4 drugs (Mfx, Bdq, Cfz, and Dlm) considered potentially dangerous because of their sum effects on QTcF.
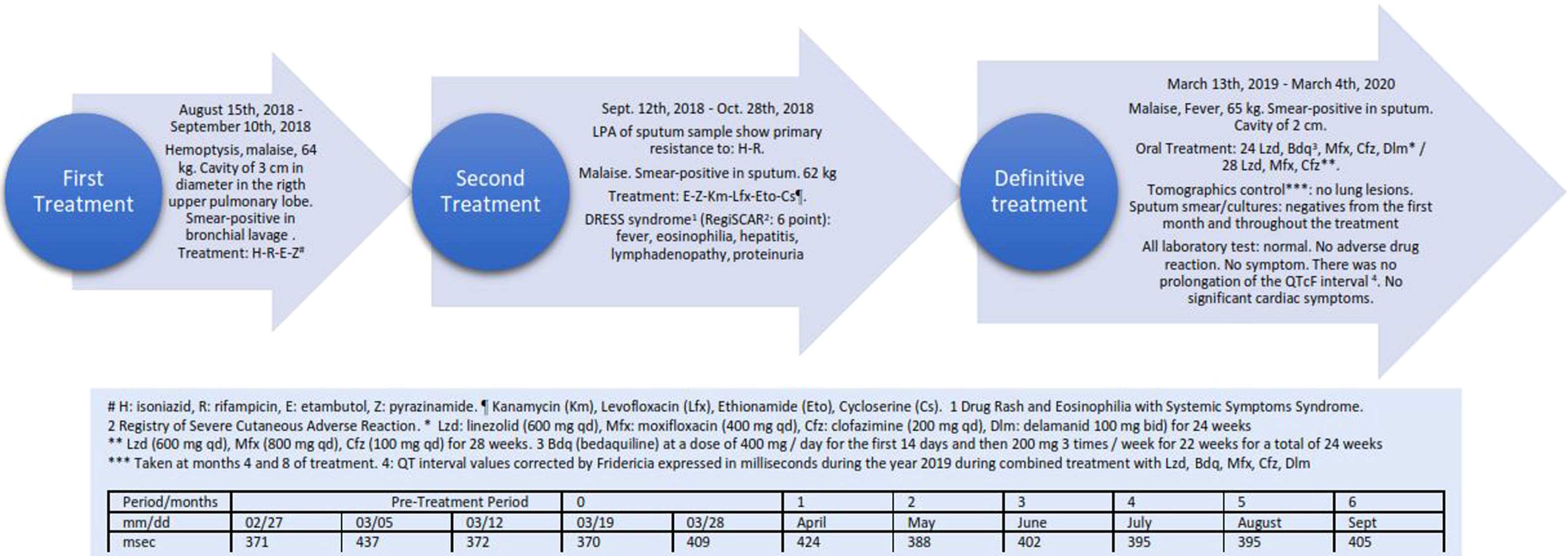
The patient was a single, nulliparous 23-year-old female with no pathological history living in Lima, Peru. At the beginning of August 2018, she presented hemoptysis and malaise and underwent a chest tomography on August 4th, 2018 showing exudative lesions in the right upper pulmonary lobe associated with a cavity of 3cm in diameter. On August 14th, 2018, bronchofibroscopy was performed, obtaining a smear-positive in bronchial lavage. Tuberculosis treatment was initiated on August 15th with isoniazid, rifampicin, ethambutol and pyrazinamide.4 The patient weighed 64 Kg at this time. This treatment was suspended on September 10th, 2018 following results of a Line Probe Assay study (Line ProbeAssay, Genotype® MTBDR Plus Version 2.0) of a sputum sample from August 16th, 2018 showing the presence of primary resistance to rifampicin and isoniazid (Gen KatG). On September 12th, 2018, a new empirical treatment was begun with ethambutol, pyrazinamide, kanamycin, levofloxacin, ethionamide and cycloserine.4 On October 16, 2018, the patient presented cervical lymphadenopathy (>3cm in diameter) and axillary lymphadenopathy, in addition to a temperature of 38.5°C and rash in the following days. On October 23, 2018, eosinophils in peripheral blood were found to be elevated (7%) and proteins were also found in a urine sample. On October 28th, 2018, antituberculosis treatment was suspended. Eosinophils in peripheral blood continued to increase to a maximum of 21% on November 19th and then normalized on January 17th, 2019. Aspartate aminotransferase (AST) values increased to 78IU/L on November 19th and normalized on December 3rd (AST: 26IU/L). Alanine aminotransferase (ALT) values reached a maximum of 152IU/L on November 10th and normalized on December 3rd (ALT: 34IU/L). The patient was diagnosed with Drug Rash and Eosinophilia with Systemic Symptoms (DRESS) syndrome, with a score of 6 points in the Registry of Severe Cutaneous Adverse Reaction (RegiSCAR) secondary to the treatment received.6
The patient was hospitalized on January 25th, 2019 with the aim of designing a new tuberculosis treatment schedule. She was stable with normal vital signs and with a weight of 65kg. Laboratory tests showed a normal blood count, mild anemia, normal renal, hepatic and cardiac function (normal echocardiogram, electrocardiogram, QTcF, and cardiac enzymes), normal glycosylated hemoglobin, and negative viral hepatitis studies (HAV, HBV, HCV). Antibiotic susceptibility testing showed sensitivity to: ethambutol, pyrazinamide, streptomycin, kanamycin, capreomycin, ethionamide, cycloserine, ethionamide, para-aminosalicylic acid (PAS), levofloxacin and Mfx. The MDR TB unit decided to use new tuberculosis drugs that had not previously been combined in Peru, and the new antituberculosis treatment was begun on March 13th, 2019. This treatment consisted of linezolid (Lzd) at a dose of 600mg/day, Mfx 400mg/day, Cfz 200mg/day, Bdq at a dose of 400mg/day for the first 14 days and then 200mg 3 times/week for 22 weeks for a total of 24 weeks, and Dlm at 100mg bid for 24 weeks. After 24 weeks of treatment, Bdq and Dlm2,7 were suspended. The patient continued to receive Lzd at the dose described. Cfz was decreased to 100mg daily from the fourth month due to changes in skin color. The dose of Mfx was increased from 400mg to 800mg daily from week 24. Throughout this period laboratory tests were normal. There was no prolongation of the QTcF interval, and no significant cardiac symptoms were observed during the entire treatment. Fig. 1 shows the results of the periodic controls of the QTcF Interval in the timeline of this case. Direct sputum smear and cultures taken monthly were negative from the first month of treatment and throughout the treatment. The 2 tomographic controls taken at months 4 and 8 of treatment showed no lung lesions.
Several aspects are of note in the present case: (1) This patient was the first Peruvian and the first Latin American patient to receive a completely oral combination of medications for the treatment of MDR tuberculosis.8 (2) This was the first Peruvian and the first Latin American case to receive a combination of 4 drugs (Mfx, Bdq, Cfz, and Dlm) considered potentially dangerous because of their sum effects on QTcF.1 (3) According to the study of Borisov et al.9 only 39 out of a total of 658 cases (6.1%) have received Bdq and Dlm consecutively or in combination, none being from Peru. In addition, of the 9 cases presenting severe cardiological events, none had received the same combination as our patient. (4) In Latin America, only Chile and Mexico together have reported 4 cases treated with the combination of Bdq and Dlm, with apparently none using Cfz and Mfx simultaneously.9 The treatment was well tolerated by our patient, with no adverse cardiovascular events like those reported in other series of patients using this combination.5,10 Cfz produced slight pigmentation of the skin that did not bother the patient. Lastly, clinical response was excellent with notable radiological improvement.
This drug combination was demonstrated to be safe and effective and well tolerated. None of the frequent adverse reactions that occur with the use of kanamycin injectable medications that are still used in Peru for the treatment of MDR tuberculosis4 were observed. Moreover, this treatment is highly effective since the sensitivity of bacteria to Bdq, Dlm and Lzd is high.11 The patient will be followed every 3 months for 2 years to record possible development of relapse. The results of this case are important for demonstrating that oral treatment of MDR tuberculosis is possible and can be carried out safely with suitable monthly clinical controls and periodic QTcF controls.
Statement of ethicsThe authors have no ethical conflicts to disclose.
FundingThe authors have not received any funding specifically for this correspondence.
Author contributionsAll authors contributed equally to this correspondence.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Delia Loayza Tamayo, Félix Alcántara Virú, and Javier Torres Valencia for their generous assistance during the treatment of the patient. The authors would like to thank Partners In Health – Perú for the donation of medicines: delamanid, clofazimine, bedaquiline.