We report a case of disseminated infection by Rhodococcus equi as the inaugural manifestation of idiopathic T-CD4+ lymphopenia. We aim to demonstrate our diagnostic and therapeutic approach and focus on the major dilemmas arising from the lack of scientific evidence regarding best clinical practice of this infection in humans.
Rhodococcus equi (R. equi) is a facultative intracelular gram-positive coccobacillus which primarily causes zoonotic infection.1,2 This bacteria is becoming an emerging opportunistic agent in humans.3 As far as we know, we present the first case of R. equi infection in a patient with idiopathic T-CD4+ lymphopenia (ICL), a rare condition defined by the repeated presence of a T-CD4+ lymphocyte count <300cells/mm3 or less than 20% of total T cells without evidence of human immunodeficiency virus (HIV) infection or other condition that might lead to decreased T-CD4+ counts.4
Case descriptionIn April 2017, a 69-year-old never smoker male presented to the emergency department with cough, left pleuritic thoracalgia and fever during the previous month. He was a retired driver who owned a farm with several animals. Past medical history included hypothyroidism treated with levothyroxine and chronic hepatitis B under entecavir. He had undergone lower left lobectomy in 2004 for a pulmonary mass, but histology of the operative specimen suggested an infectious etiology and the patient received no further treatment or follow-up.
On admission, he had peripheral oxygen saturation of 97% on room air and decreased respiratory sounds in the left pulmonary field.
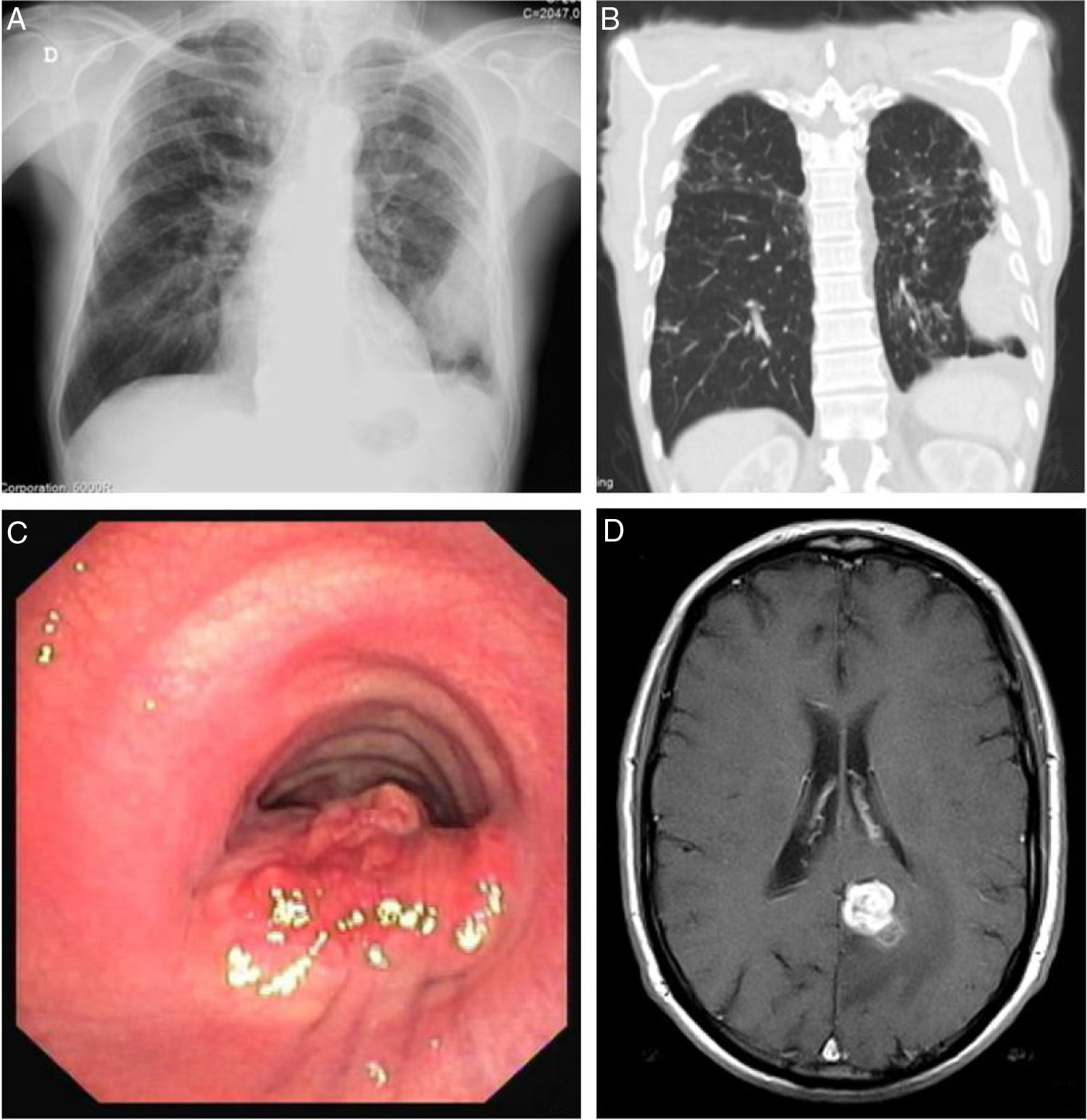
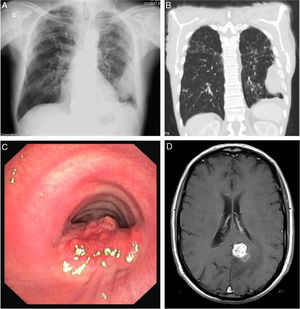
Blood test revealed white blood cells count of 10030/μL (83.3% neutrophils and 7.5% lymphocytes) and C-reactive protein of 14.7mg/dL. Chest X-ray revealed a pleural-based consolidation in the left pulmonary field (Fig. 1A) which was characterized by contrast-enhanced chest computed tomography (CT) showing a 67×41×24mm mass on the periphery of the left upper lobe (LUL) with heterogeneous contrast uptake and hypodense areas suggestive of necrosis (Fig. 1B).
Sites of Rhodococcus equi infection. (A) Lung as a consolidation in the left pulmonary field on posteroanterior X-ray; (B) lung as a mass on the periphery of the left upper lobe on chest computed tomography; (C) trachea as a protruding lesion leading to obstruction of about 50% of the lumen; (D) brain as an abscess in the left parasagital occipitoparietal location on magnetic resonance.
The diagnosis of necrotizing pneumonia was established and the patient was hospitalized. Urine was negative for pneumococcal and Legionella antigens. Blood cultures were collected. Intravenous amoxicillin/clavulanic acid and azithromycin were started. Bronchofibroscopy and bronchoalveolar lavage (BAL) were conducted without endobronchial lesions. R. equi was isolated in both blood cultures and BAL. Antimicrobial susceptibility test (AST) showed sensitivity to imipenem and levofloxacin and intermediate sensitivity to ceftriaxone and ciprofloxacin. Treatment was adjusted for a combination of rifampicin and levofloxacin. Although sensitivity to rifampicin could not be tested, it was included due to its intracellular action and because it is a first choice drug.3,5 The patient underwent transthoracic aspiration biopsy of the LUL lesion that excluded malignancy and showed signs of R. equi infection, namely, histiocyte foci with granulomatous configuration, necrosis and coccoid elements in the macrophage cytoplasm. The diagnosis of necrotizing pneumonia by R. equi with hematogenous dissemination was established. Central nervous system, cardiac, abdominal and cutaneous involvement were excluded. HIV and human T-lymphotropic infections were ruled out. Further extensive work-up to detect any other immunodeficiency condition was made leading to the diagnosis of ICL with an initial T-CD4+ lymphocyte count of 28cells/μL.
The patient was discharged after two weeks of intravenous treatment with rifampicin and levofloxacin and instructed to continue oral antibiotic treatment at home.
After one month, blood cultures were negative and radiological improvement was documented with a significant reduction in the LUL mass. After six months of treatment, bronchofibroscopy revealed persistence of R. equi in BAL, albeit with significant reduction in the number of colonies.
In April 2018, nearly one year after starting treatment, the patient developed stridor. Rigid bronchoscopy showed obstruction of the lumen of the lower third of the trachea in about 50% due to a protruding lesion (Fig. 1C) in which endoscopic treatment was conducted. Tracheal lesion biopsy revealed chronic inflammation with intracellular bacteria, malakoplakia and no signs of malignancy. Relapse of R. equi infection was assumed based on these histopathological findings, but since microbiological isolation was not obtained it was not possible to determine AST. Other potential recurrence sites were investigated leading to the diagnosis of an asymptomatic small brain abscess in magnetic resonance without indication for surgical treatment (Fig. 1D). A new antibiotic regimen was started with ertapenem, gentamycin and linezolide according to the literature.3,5 One-year treatment was proposed and a central line was placed allowing outpatient parenteral treatment.
On July 2018 linezolid was replaced by clarithromycin due to myelotoxicity and on January 2019 gentamycin was replaced by doxycycline due to nephrotoxicity and ototoxicity.
On July 2019 the patient was under ertapenem (14 months), clarithromycin (12 months) and doxycycline (7 months) with a favorable clinical response, resolution of cerebral abscess and no evidence of infection recurrence. These antibiotics were discontinued and it was decided to start prophylactic treatment with cotrimoxazole. So far the patient has been under surveillance and has not undergone any further treatment.
DiscussionDiagnostic approach of R. equi infection requires high clinical suspicion. Multidisciplinary discussion with microbiology expert is of vital importance since R. equi may be misdiagnosed as a contaminant or other bacteria. Due to its partial alcohol-acid resistance, R. equi infection may lead to misdiagnosis of mycobacteriosis.2,6R. equi infection may also mimic malignancy since pulmonary nodules or masses may be the radiological manifestation.3 We believe that the lesion treated with lobectomy in 2004 could already correspond to infection by R. equi, since at that time the patient had contact with animals and signs of infection by a supposed atypical mycobacteria on the operative specimen were found. A radical treatment such surgery, the insidious course of the disease and continued contact with animals could explain why the infection only manifested again over ten years later.
R. equi infection prompted the investigation of an immunosuppressive condition leading to the inaugural diagnosis of ICL. T-CD4+ cells, mainly Th1 cells, are involved in acquired resistance against facultative intracellular bacteria and increase bactericidal activity of macrophages by producing gamma interferon.7 Th1 response seems to be crucial in pulmonary clearance of R. equi.3 Recombinant human IL-7 may be a promising therapeutic intervention in ICL, leading to an increase in T-CD4+ cells in both peripheral blood and tissues.8 It may be questionable to assume the diagnosis of ICL since opportunistic infections may themselves lead to a depression of T-CD4+ cell count.9 Because low T-CD4+ cell count persisted for over two years after the first analysis and no apparent cause had been determined, the diagnosis of ICL was made by exclusion.
Regarding treatment there are no consensus guidelines. Because acquired resistances are of concern, combination treatment is recommended with two or three antibiotics to which the agent is susceptible and at least one drug should have good intracellular activity.3 First line drugs in humans include rifampicin, levofloxacin, erythromycin, imipenem, vancomycin and aminoglycosides.5 For immunocompromised patients at least six months of antibiotic therapy are advised.10 The placement of a central venous catheter allows intravenous treatment on an outpatient basis, reducing risks of nosocomial infection and costs. Side-effects from multi-drug treatment over such a long period of time may be a concern and should be closely monitored. Antibiotics withdrawal and substitution may be needed, making R. equi infection treatment a dynamic process.
Relapse may occur requiring close surveillance. We scheduled hospital medical consultation whenever the patient presented any complaint and every three months with hemogram, serum biochemistry, blood and sputum cultures and chest computed tomography. After relapse with a brain abscess, brain magnetic resonance was performed every three months until its resolution. No further bronchoscopy was performed following endoscopic treatment of tracheal pseudotumor as the patient refused further invasive examinations and remained asymptomatic.
FundingThis case report did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors have no conflicts of interest to declare.