While the association between handgrip strength and all-cause mortality is more deeply explored, no previous studies have been specifically focused on handgrip strength and respiratory disease mortality. The purpose of the study was to investigate the association between handgrip strength and respiratory disease mortality in a large representative sample.
MethodsIndividuals aged 50 or over from 27 European countries and Israel participated in this longitudinal study. Data on handgrip strength and all-cause and respiratory disease mortality were retrieved from the Survey of Health, Ageing and Retirement in Europe (SHARE) waves 1, 2, 4, 5, 6 and 7. We estimated the sub hazard ratios (SHRs) for respiratory disease mortality using a Fine-Gray sub-distribution method with both time-varying exposure and covariates and mortality due to other causes as competing risk. Furthermore, we assessed dose‐response associations of handgrip strength (modelled as a continuous exposure) with respiratory disease mortality using restricted cubic splines and estimated hazard ratios (HRs).
ResultsWe included 60,883 men and 74,904 women with a mean age of 63.6 (SD 9.7) years at study entry. During a median (interquartile range) of 7.4 years of follow-up 565 (0.4%) participants died due to respiratory diseases. The increase of 1 single kg of handgrip strength showed a 6% incidence reduction on respiratory disease mortality (SHR, 0.94; 95%CI, 0.92-0.96) after adjusting for potential confounders. Furthermore, each kg increase of handgrip strength reduced respiratory disease mortality risk in a dose-response fashion and a significant threshold for values of 41 kg (HR, 0.49; 95%CI, 0.26-0.92) and higher was identified.
ConclusionsHigher handgrip strength is associated with lower mortality due to respiratory disease. Intervention studies are needed to determine whether strength training in respiratory disease patients can prevent premature mortality.
Respiratory disease is the third main cause of mortality worldwide and the incidence keeps increasing on both disability adjusted life years (DALYs) and mortality.1–3 The most common respiratory diseases that are the leading cause of mortality are chronic respiratory diseases (CRD), including Chronic Obstructive Pulmonary Disease (COPD) as the most prevalent, followed by asthma.2,3 For instance, in 2017, the estimated deaths due to CRD were 3.91 million, which composed 7% of all deaths worldwide.3 Therefore, respiratory diseases are a major issue in public health that needs to be tackled.
Skeletal muscle dysfunction appears with aging4 and is common and exacerbated in CRD.5,6 This dysfunction has a great negative impact on physical performance, leading to disability,7 and importantly, is related to increased mortality risk.5,8 However, among health care providers, a lack of awareness regarding the critical role of skeletal muscle dysfunction still prevails.5,9 An easy-to-use and objective tool to evaluate whether skeletal muscle dysfunction occurs, is measuring handgrip strength. Apart from being an indicator of overall muscle strength,10–12 handgrip strength has been widely used as a biomarker of health status as well as to predict mortality.12,13
While the association between handgrip strength and all-cause mortality is more deeply explored,13–15 no previous studies have been specifically focused on handgrip strength and respiratory disease mortality, and thus its relationship remains unclear. For instance, a cohort study among 502 293 participants from the UK investigating all-cause mortality as well as disease specific mortality reported that lower handgrip strength was related to higher all-cause mortality as well as to cause-specific mortality from respiratory diseases.14 However, previous data from the Prospective Urban Rural Epidemiology (PURE) study,15 where an association between handgrip strength and all-cause mortality was found, no significant association between handgrip strength and hospital admission due to respiratory disease was revealed. In addition, a recent meta-analysis could not determine causality between disease-specific mortality, including respiratory diseases, and handgrip strength.13 Notably, none of the previous studies mentioned above included a sample involving a large number of European countries. Since differences in cultural, environmental and lifestyle factors might influence the results,14 new studies with more representative data from this specific continent are needed. Furthermore, understanding the potential causal and confounding pathways between handgrip strength and respiratory disease mortality in a large representative sample of subjects could help to clarify the potential role of this measure as a clinical tool for mortality risk estimation of respiratory disease. Because higher levels of handgrip strength might relate to both chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD) and acute respiratory diseases such as pneumonia,16,17 a potential joint effect of handgrip strength could be considered. The main objective of this study was to investigate the association between handgrip strength and respiratory disease mortality using only adults free from prior or current known respiratory disease. We hypothesised that higher handgrip strength was prospectively associated with reduced respiratory disease mortality risk.
Material and methodsPopulationThe present study included data from waves 1, 2, 4, 5, 6, and 7 from the Survey of Health, Ageing and Retirement in Europe (SHARE), a biannual survey recruiting individuals aged 50 or older residing in 27 European countries and Israel.18,19 We did not consider wave 3 in the current study because data on the exposure of interest (i.e., handgrip strength) was not available for that wave. Representativeness of SHARE waves is assured using a multi-stage stratified sampling design in which countries are divided into different strata according to their geographical area. Municipalities or zip codes within these strata served as primary sampling units.20 Data collection was conducted through home computer-assisted personal interviews and measures from February 2004 to January 2019. SHARE data were collected using ex-ante harmonised interviews, and new respondents were added in each wave to compensate for the attrition bias due to losses.20 Only participants aged 50 years or older and that were free from any current or prior known chronic or acute respiratory disease were included in the current study (n=135 787). Missing values in any study variable from included participants were estimated using multiple imputation (35%). Fig. 1 shows more descriptive details of the study sample. The present study received the approval of the Ethics Committee of Research in Humans of the University of Valencia (1510464) and was reported according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).21
Handgrip strength (exposure)Handgrip strength was measured twice for each hand using a handheld dynamometer (Smedley, S Dynamometer, TTM, Tokyo, 100 kg). Following the SHARE protocol, participants were instructed to set their elbow in a 90° angle flexion while either standing or sitting, keeping a neutral wrist position, and upper arm vertically set against the trunk. Measures were taken in participants´ homes at the time of conducting the survey. The same trained interviewers that conducted the in-person home computer-assisted personal interviews assisted with the handgrip strength measurement and verbally encouraged participants with standardised instructions to squeeze the dynamometer with maximum effort for a few seconds. Handgrip strength was defined as the maximum value of either hand.
All-cause and respiratory disease mortality (outcome)Participants were followed throughout the study period to determine whether they were dead or alive. In case of death, information regarding both date and cause of death was retrieved from a proxy interview. A standardized end-of-life interview conducted with proxy-respondents ex post served to ascertain the cause of death. Less than 1% of the eligible participants had missing values on cause of death, which was due to either refusal of the proxy to disclose such information or lack of information. More information on mortality cause has been provided elsewhere.19 For specific causes of mortality, the interviewers asked the following question: “What was the main cause of respondent's death?” Possible answers comprised cancer, heart attack, stroke, other cardiovascular disease related illnesses (heart failure and arrhythmia), respiratory, digestive, or severe infectious disease, and other causes. For the purpose of this study, participants were categorised into 0 (alive), 1 (death due to respiratory disease), and 2 (death due to other causes).
CovariatesBased on literature,22,23 we explored potential causal and confounding pathways using a directed acyclic graph (eFig. 1). Please see Appendix A for covariates explanation.
Statistical analysesWe conducted all statistical analyses in Stata version 16.1 (StataCorp, Texas, USA). We used a Fine-Gray sub-distribution method model with both time-varying exposure and covariates to estimate the subhazard ratios (SHRs) for respiratory disease mortality, which accounted for competing risk (i.e., mortality due to other causes). Time-on-study in months was used as the timescale.
We examined the proportional hazards assumption by testing interactions with log(time) using stphplot command and found no evidence of assumption violation. After assessing interactions between handgrip strength and all the covariates, no significant interaction was detected. Two models were tested; a model including gender and age at the time of the interview as confounder (Model A) and a fully adjusted model (Model B) that included covariates of Model A plus country, education, body mass index, smoking, and physical inactivity as confounders. All the analyses accounted for the survey design and were weighted according to each country population. The results were visualized as forest plots. In addition, we assessed the dose‐response associations of handgrip strength (modelled as a continuous exposure) with respiratory disease mortality using restricted cubic splines to allow for potential non‐linearity and obtained hazard ratios (HRs). For this analysis, we trimmed observations less than 5% and greater than 95% of the distribution and pre-specified knots placed at the 5th, 25th, 50th, 5th, and 95th percentiles of the exposure distribution.24 We assumed linearity for values below the 5th percentile and for values above the 95th percentile. Departure from linearity was assessed by a Wald test examining the null hypothesis that the coefficient of the fifth spline was equal to zero. Results are reported as either SHRs or HRs with 95% CIs and levels of significance were set at p < 0.05. Sensitivity (eFigs. 2, 3) and robustness analyses (eFig. 4) were conducted and are explained at Appendix B.
ResultsDemographicsThe final sample included 60,883 (44.8%) men and 74,904 (55.2%) women with a mean age of 63.6 (SD 9.7) years at study entry (Table 1). During a median (interquartile range) of 7.4 years of follow-up (5.7-12.8) and 1 013 089 person-year, 565 (0.4%) participants died due to respiratory diseases.
Characteristics of participants at study entry.
| N = 135 787 | n (%) | Mean (SD) |
|---|---|---|
| Age (y) | 63.6 (9.7) | |
| Sex | ||
| Men | 60883 (44.8) | |
| Women | 74904 (55.2) | |
| Body Mass Index (kg/m2) | ||
| Underweight (<18.5 kg/m2) | 1806 (1.3) | |
| Normal (18.5-<25 kg/m2) | 47471 (35.0) | |
| Overweight (25-<30 kg/m2) | 56365 (41.5) | |
| Obese (≥30 kg/m2) | 30145 (22.2) | |
| Educationa | ||
| None | 6138 (4.5) | |
| Primary | 25745 (19.0) | |
| Lower secondary | 24102 (17.7) | |
| Upper secondary | 46195 (34.0) | |
| Post-secondary non-tertiary | 5988 (4.4) | |
| First stage of tertiary | 26696 (19.7) | |
| Second stage of tertiary | 923 (0.7) | |
| Current smoking habit | ||
| No | 97196 (71.6) | |
| Yes | 38591 (28.4) | |
| Physical inactivity | ||
| No | 114876 (84.6) | |
| Yes | 20911 (15.4) | |
| Country | ||
| Austria | 6225 (4.6) | |
| Belgium | 9583 (7.1) | |
| Bulgaria | 1942 (1.4) | |
| Croatia | 2851 (2.1) | |
| Cyprus | 1200 (0.9) | |
| Czech Republic | 8410 (6.2) | |
| Denmark | 5682 (4.2) | |
| Estonia | 7666 (5.6) | |
| Finland | 1974 (1.4) | |
| France | 8106 (6.0) | |
| Germany | 8596 (6.3) | |
| Greece | 6411 (4.7) | |
| Hungary | 3045 (2.2) | |
| Ireland | 1005 (0.7) | |
| Israel | 3907 (2.9) | |
| Italy | 8364 (6.2) | |
| Latvia | 1685 (1.2) | |
| Lithuania | 1987 (1.5) | |
| Luxembourg | 2116 (1.6) | |
| Malta | 1241 (0.9) | |
| Netherlands | 6341 (4.7) | |
| Poland | 6188 (4.5) | |
| Portugal | 2143 (1.6) | |
| Romania | 2055 (1.5) | |
| Slovakia | 1966 (1.5) | |
| Slovenia | 5382 (4.0) | |
| Spain | 8656 (6.4) | |
| Switzerland | 4507 (3.3) | |
| Sweden | 6543 (4.8) | |
| Handgrip strength (kg) | 31.8 (12.0) |
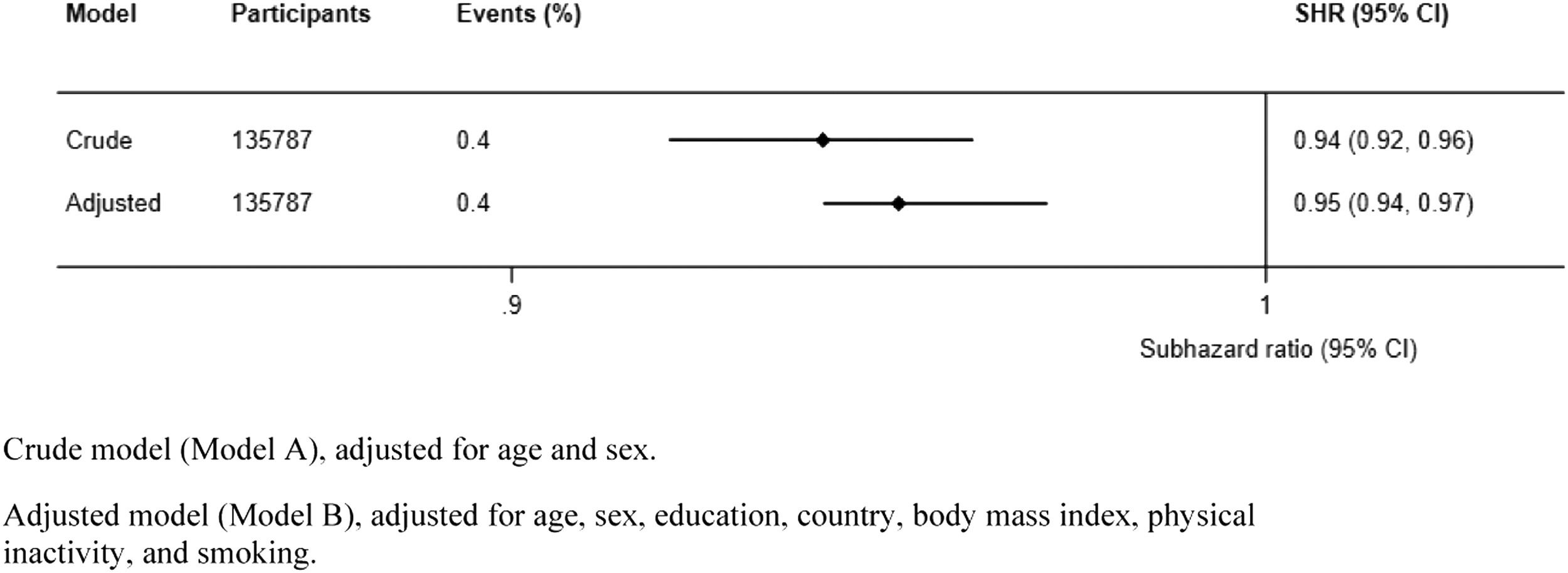
Results from the model adjusted for sex and age only (Model A) showed that one single increase of a handgrip strength kilogram showed an incidence reduction of respiratory disease mortality of 6% at any time (SHR, 0.94; 95% CI, 0.92-0.96) (Fig. 2). The observed associations were consistent in the fully adjusted model (Model B), which displayed a respiratory disease mortality incidence reduction of 5% (SHR, 0.94; 95% CI, 0.92-0.96) (Fig. 2).
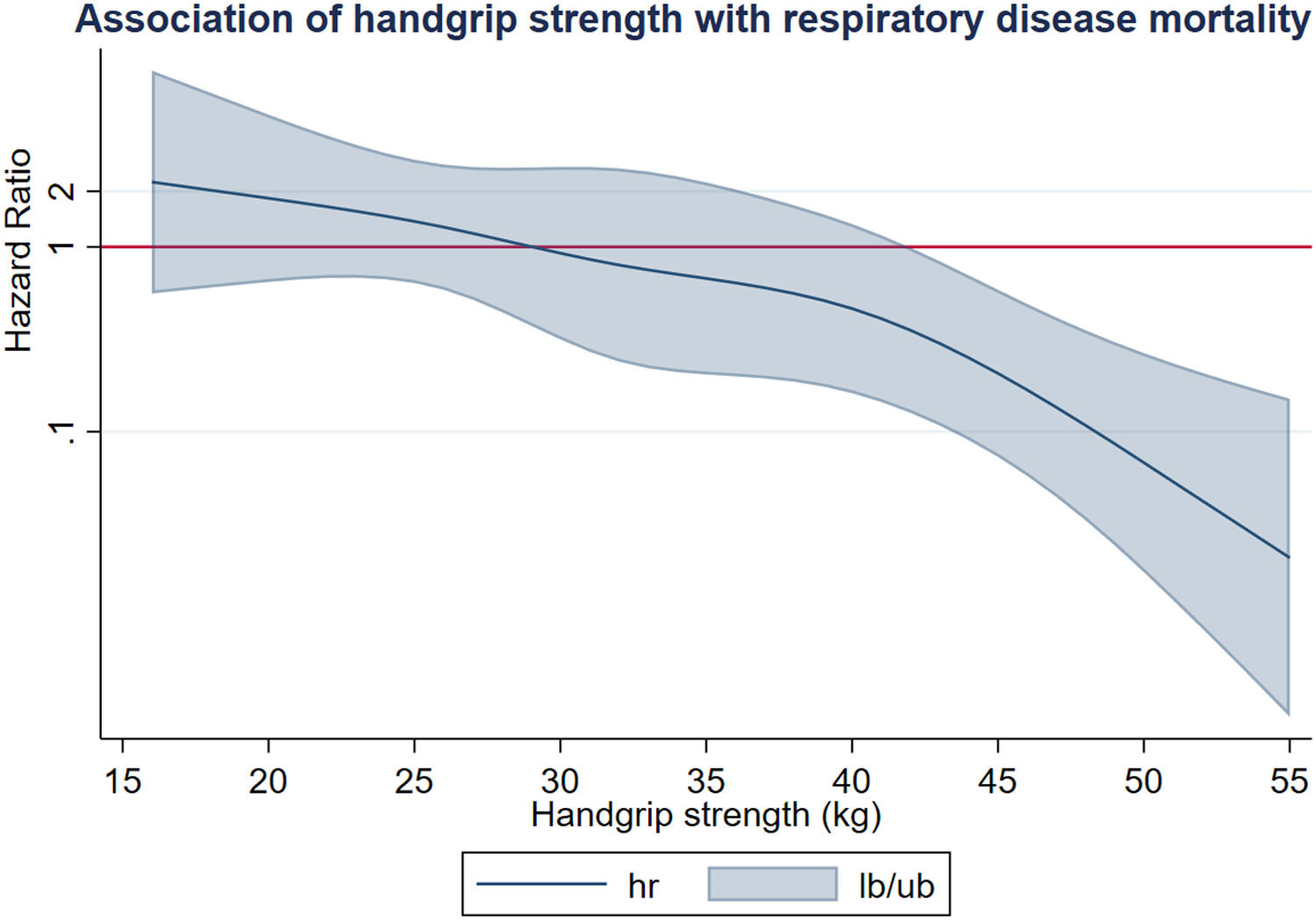
Analyses using spline modelling showed a linear dose-response fashion with a respiratory disease mortality risk reduction for each kg increase of handgrip strength and a significant threshold for values of 41kg and higher (SHR, 0.49; 95% CI, 0.26-0.92) (Fig. 3).
Additional sensitivity analysesResults of sensitivity analyses excluding deaths occurring within the first two years of follow-up did not substantially differ from those of the main analysis (eFig. 2). However, spline modelling using the fully adjusted model (Model B) exhibited slight variations of the dose-response linear patterns (eFig. 3).
DiscussionIn this large-scale study including 135 787 subjects, each one kg increase of handgrip strength was prospectively associated with a substantial respiratory disease mortality reduction.
Our result endorses previous findings, where higher handgrip strength was inversely related to mortality.13–15 However, despite the association found between all-cause mortality and handgrip strength, in a large-scale longitudinal study conducted in 17 countries, no association between handgrip strength and hospital admission due to respiratory disease was found.15 Nevertheless, hospital admission is the result of acute respiratory deterioration, and since the underlying mechanism of CRD differs from that of an acute exacerbation5,25 a different effect on skeletal muscle dysfunction could be expected, which could have caused the difference in findings. In addition, the median follow-up time at the aforementioned study was merely four years whereas in our study, the median follow up was 7.4 years. Because skeletal muscle dysfunction is more extensive as the disease progresses,5,26 this might explain the controversial results. The meta-analysis by Lee showing an inverse association of handgrip strength with all-cause mortality,13 included one study that did specifically investigate the association between handgrip strength and respiratory disease.27 This is a 24-year follow-up study from the UK, where a negative association was found between handgrip strength and respiratory disease mortality. However, after adjusting for potential confounders (height, smoking, social class, physical activity, diagnosed disease at baseline, calorie intake, reported weight loss, and the measures of body composition) the association was not statistically significant. Since that study included only participants from the UK and as there are differences in cultural and environmental factors other than adjusted for, like the use of alcohol or diet preferences,28,29 this might explain the difference in the results. Interestingly, in a more recent meta-analysis investigating the association between handgrip strength and mortality, morbidity and health related quality of life in patients with COPD, a small to moderate association between handgrip strength and mortality was found,30 however within the included studies contrasting evidence was present. For instance, authors of a longitudinal cohort study claimed that they could not confirm associations between handgrip strength and respiratory disease mortality that are previously found in cross-sectional studies.31 However, this longitudinal study only included 194 patients with COPD that were followed-up and it has been shown that the disease progression and the procedure of skeletal muscle dysfunction within COPD patients are very variable.5,32 A possible mechanism explaining the variability of skeletal muscle dysfunction in patients with COPD is that there are three different domains in which muscle dysfunction is present: a clinical/functional domain, a metabolic domain, and an anatomical domain, and all of these domains can get dysfunctional in an independent way and therefore cause a variable and unique manifestation of skeletal muscle dysfunction.5,32 The most common CRD that is the leading cause of mortality is COPD,2,3 and therefore causing a high variability of skeletal muscle dysfunction in our group of participants. Nevertheless, our longitudinal study includes a large heterogeneous population and may therefore capture the high variability of muscle dysfunction within the participants.
We found a linear association for respiratory disease mortality risk reduction and an increase of handgrip strength, indicating higher muscle strength levels are consistently associated with lower respiratory disease mortality risk. In fact, we found that an increase of 1 kilogram of handgrip strength was associated with a respiratory disease mortality reduction of 5%. It is likely that handgrip strength is simply a proxy measures of overall muscle strength. Thus, a training program aiming to improve overall strength levels might be beneficial for patients with respiratory disease. We suggest that by training and increasing handgrip strength we can intercept the clinical/functional domain as a mechanisms accounting for skeletal muscle dysfunction seen for patients with respiratory disease.5 The clinical/functional domain is about the endurance and strength of the muscle, and this can be improved by training.33 However, this should be tested in large-scale randomized trials before any firm recommendations can be provided.
Although several validated tests assess the systemic involvement of CRDs and therefore obtain a reasonable measurement of muscle dysfunction,34 the handgrip measurement offers some advantages, particularly because it is easy to perform, is low cost, and requires very little equipment. In addition, it can be used in contexts in which patients cannot walk or make significant physical effort, such as in the 6 min walk test or have difficulty performing strength manoeuvres (e.g., knee flexors or extensors).35
The key strengths of the present study are the use of a large and representative sample from 28 countries with an objective measure of handgrip strength. Another critical strength is the use of time-varying handgrip strength and covariates in our modelling strategy, which reduces the possibility of bias of our estimates, which also accounted for competing risks of mortality due to other causes). Furthermore, repetitive measures of handgrip strength served to model the continuous dose-response association of handgrip strength with respiratory disease mortality, which is critical to identifying both thresholds and shape of the dose-response association. Furthermore, we also took measures to minimise the chance of reverse causation (i.e., lower handgrip strength as result of the course of the disease) by removing all the events that occurred within the first 2 years of follow up. On the other hand, there are several limitations to be considered in the present study. First, due to the number of participants with missing values concerning time-varying covariates, we imputed a substantial number of values, which might lead to some measuring bias, although complete case analyses scarcely differed from the main analyses. Second, due to the low number of events, the estimates for the fully adjusted model (Model B) are considerably unstable for sensitivity analyses regarding dose-response associations. Third, the participation rate at baseline was moderate (56%), which possibly increased the risk of selection bias. However, such losses are compensated through the use of refresher samples.19 Also, there is the chance of some attrition bias which might hamper the accuracy of our estimations, but the average retention rate in SHARE (81%) importantly reduces such possibility.19 For this reason, we included a weight variable in the analyses to compensate both non-response and attrition. Fourth, the use of a proxy relative for assessing respiratory disease mortality might lead to a certain degree of misclassification in that variable. Although prior research has observed that a relative proxy is a robust substitute to identify death status in adult populations when information on death is not available,36 information on specific mortality cause may be subject to a higher risk of misclassification bias. Fifth, because individuals with other prior or current chronic respiratory disease conditions other than COPD could not be identified, several of the mortality outcomes of the present studies might be more related to other prior critical chronic respiratory conditions such as asthma instead of lower handgrip strength.2 Lastly, because the prior or current COPD condition is self-reported, certain degree of measurement bias concerning this variable is still plausible
ConclusionsLower handgrip strength is associated with higher mortality due to respiratory disease. Improving handgrip strength by one single kilogram in patients with respiratory disease, could potentially reduce their mortality by 5%.
Ethical responsibilitiesNone declared.
FundingRLB is supported by the European Union - Next Generation EU.
This paper uses data from SHARE Waves 1, 2, 4, 5, 6, and 7 (DOIs: 10.6103/SHARE.w1.710, 10.6103/SHARE.w2.710, 10.6103/SHARE.w4.710, 10.6103/SHARE.w5.710, 10.6103/SHARE.w6.710, 10.6103/SHARE.w7.711, see Börsch-Supan et al. for methodological details20.
The SHARE data collection has been funded by the European Commission through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006-062193, COMPARE: CIT5-CT-2005-028857, SHARELIFE: CIT4-CT-2006-028812), FP7 (SHARE-PREP: GA N°211909, SHARE-LEAP: GA N°227822, SHARE M4: GA N°261982, DASISH: GA N°283646) and Horizon 2020 (SHARE-DEV3: GA N°676536, SHARE-COHESION: GA N°870628, SERISS: GA N°654221, SSHOC: GA N°823782) and by DG Employment, Social Affairs & Inclusion. Additional funding from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the U.S. National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064, HHSN271201300071C) and from various national funding sources is gratefully acknowledged (see www.share-project.org).
Self-reported age and sex, country of residence at the time of the interview, education, body mass index, smoking and physical inactivity were identified as potential confounders. Education was self-reported by participants and thereafter coded using the 1997 version of the International Standard Classification of Education.37 Body mass index was calculated from self-reported height and weight and subsequently grouped into four categories according to standards proposed by World Health Organization (WHO).38 Smoking habits were assessed through the following question: “Have you ever smoked cigarettes, cigars, cigarillos, or a pipe daily for a period of at least one year?”. Finally, physical inactivity was determined through two questions: “How often do you engage in vigorous physical activity such as sports, heavy housework, or a job that involves physical labour”, and “How often do you engage in activities that require a moderate level of energy such as gardening, cleaning the car, or going for a walk?”. Participants selecting the option of “Hardly ever, or never” in the two questions were considered to be physically inactive.20
To minimize the potential influence of reverse causality, we conducted sensitivity analyses excluding participants who died within the first 2 years of follow-up for both imputed (eFig. 2) and complete-case analyses (eFig. 3). Moreover, we conducted complete case analyses of the dose-response association between handgrip strength and respiratory disease in the fully adjusted model (Model B) to check the robustness of the results (eFig. 4). All these models also accounted for mortality due to other causes as competing risk.