International guidelines recommend endurance (ET) and strength training (ST) in patients with chronic respiratory diseases (CRDs), but only provide rough guidance on how to set the initial training load. This may unintentionally lead to practice variation and inadequate training load adjustments. This study aimed to develop practical recommendations on tailoring ET and ST based on practices from international experts from the field of exercise training in CRDs.
Methods35 experts were invited to address a 64-item online survey about how they prescribe and adjust exercise training.
ResultsCycling (97%) and walking (86%) were the most commonly implemented ET modalities. Continuous endurance training (CET, 83%) and interval endurance training (IET, 86%) were the frequently applied ET types. Criteria to prescribe IET instead of CET were: patients do not tolerate CET due to dyspnoea at the initial training session (79%), intense breathlessness during initial exercise assessment (76%), and/or profound exercise-induced oxygen desaturation (59%). For ST, most experts (68%) recommend 3 sets per exercise; 62% of experts set the intensity at a specific load that patients can tolerate for a range of 8 to 15 repetitions per set. Also, 56% of experts advise patients to approach local muscular exhaustion at the end of a single ST set.
ConclusionsThe experts´ practices were summarized to develop practical recommendations in the form of flowcharts on how experts apply and adjust CET, IET, and ST in patients with CRDs. These recommendations may guide health care professionals to optimize exercise training programs in patients with CRDs.
Patients with chronic respiratory diseases (CRDs) perceive various limitations of exercise capacity, which go far beyond (exertional) breathlessness. Peripheral muscle weakness and associated physical inactivity accelerate physical deconditioning and amplify exercise-induced breathlessness and peripheral muscle discomfort.1 The beneficial effects of exercise training (either as a standalone intervention or as part of a pulmonary rehabilitation (PR) program) have been well documented in patients with CRDs.2-4 Thus, exercise training has been established as a key component of non-pharmacological treatment options in CRDs.5,6 However, when it comes to practical recommendations on how to prescribe exercise training in patients with CRDs, there is only scarce information available in the international respiratory societies’ official statements and guidelines (Tables 1-2).5,7,8 This lack of information is even more noticeable regarding how exercise training should be adjusted and progressed during an ongoing exercise training program. Therefore, we collected the experiences of multiple international experts from the field of exercise training in CRDs on how they initially set and subsequently adjust exercise training workloads in patients with CRDs.
Overview of recommendations to prescribe and adjust continuous and interval endurance training in patients with chronic respiratory diseases including experts’ practices from the current survey.
| Continuous endurance training (CET) | ACCP/AACVPR 2007 8 | BTS 2013 7 | ATS/ERS 2013 5 | Evidence from the literature (n=13 studies) 1 Meta-analysis 10 | Expert-based practices from the current survey 2022 |
|---|---|---|---|---|---|
| Modality | Walking or cycling | Walking or cycling | Walking (treadmill or ground-based) or cycling (cycle ergometer) | Stationary cycling | Walking (treadmill or ground-based) or cycling (cycle ergometer) |
| Frequency | Not stated | 2-3 days per week (minimum) | 3-5 days per week | 2-6 days per week | 2-3 days per week (outpatient)5-7 days per week (inpatient) |
| Intensity | 60-80% of peak work rate | >60% of peak work rate | >60% of peak work rate | 50-80% of peak work rate | 60-70% of peak work rate |
| Duration | Not stated | 30-60 minutes per session | 20-60 minutes per session | 20-47 minutes per session | 30-40 minutes per session |
| Dyspnoea/Leg discomfort (10-point Borg scale) | Not stated | Not stated | 4-6 | 4-5 | 4-6 |
| Criteria for workload progression | Not stated | Not stated | Not stated | - Mainly when symptoms on the Borg scale are <3-4- increase the intensity by 5-10% of baseline workload- total workload increments weekly or monthly | When symptoms on the Borg scale are ≤3:1) Increase intensity and2) Increase duration, both according to symptomsWhen symptoms on the Borg scale are >4:Increase duration up to 30-40 minutes, if toleratedIncrease intensity, according to symptomsSee the flowchart for detailed information (Figure 1) |
| Interval endurance training (IET) | ACCP/AACVPR 2007 8 | BTS 2013 7 | ATS/ERS 2013 5 | Evidence from the literature (n=13 studies) 1 Meta-analysis 10 | Expert-based practices from the current survey 2022 |
|---|---|---|---|---|---|
| Modality | Not stated | Walking or cycling | Typically stationary cycle based | Stationary cycling | Typically cycle based |
| Frequency | Not stated | 2-3 days per week (minimum) | 3-5 days per week | 2-6 days per week | 2-3 days per week (outpatient)5-7 days per week (inpatient) |
| Intensity | Not stated | Not stated | Not stated | 80-100% of peak work rate for the active period and typically complete rest in the passive period | 80-100% of peak work rate |
| Duration | Not stated | 30-60 minutes per session, interval duration not stated | 20-60 minutes per session; interval duration not stated | 20-45 minutes per session, most common rate 1:1 with 30 seconds per interval | 20-40 minutes per session;The most common mode is 1:1 with 30-60 seconds per interval |
| When to apply interval instead of continuous endurance training (CET) | Not stated | The choice of interval or continuous training will be down to the patient and/or therapist‘s preference | Not stated |
|
|
| Criteria for workload progression | Not stated | Not stated | Not stated | - Mainly when symptoms for the BORG scale are <3-4- increase the intensity by 10-20% from the baseline workload- total workload increment weekly | When symptoms on the Borg scale are ≤3:Increase intensityReduce the duration of the recovery periodWhen symptoms on the Borg scale are >4:Increase total duration up to 20-40 minutes, if tolerated
|
Abbreviations: ACCP – American College of Chest Physicians, AACVPR – American Association of Cardiovascular and Pulmonary Rehabilitation, BTS – British Thoracic Society, ATS – American Thoracic Society, ERS – European Respiratory Society, CET – continuous endurance training.
Overview of recommendations to prescribe and adjust strength training in patients with chronic respiratory diseases including experts’ practices from the current survey.
| Strength Training (ST) | ACCP/AACVPR 2007 8 | BTS 2013 7 | ATS/ERS 2013 5 | Evidence from the literature (n=11 RCTs) 2 Meta-Analyses 1112 | Expert-based recommendations from the current survey 2022 |
|---|---|---|---|---|---|
| Modality | Machine weights, free weights, elastic resistance bands, and lifting the body against gravity | Not stated | Not stated | Strength training machines, dumbbells, elastic tubes, or bodyweight | Strength training machines, dumbbells, elastic tubes, or bodyweight |
| Frequency | 2-3 days per week | 2 days per week (minimum) | 2-3 days per week | 2-3 days per week | 2-3 days per week (outpatient)5-7 days per week (inpatient) |
| Load | Not stated | Not stated | 60-70%1RM or 8-12 RM | 40-90% 1RM | 8-15 RM at an intensity that evolves `local muscular exhaustion´ |
| Duration | Not stated | 2-4 sets of 10-15 repetitions | 1-3 sets of 8-12 repetitions | 2-4 sets of 5-15 repetitions | 3 sets à 8-15 repetitions |
| Criteria for workload progression | Not stated | The load chosen should be individualized and progressed once all sets can be completed with the selected weight | When an individual can perform the current workload for 1 or 2 sets over the desired number of 6 to 12 repetitions, on 2 consecutive training sessions the load should be increased |
| When patient discontinued strength training exercise <8 repetitions:1) Decrease the load until patients reach `momentary muscular failure´ between 8 to 15 repetitions2) Decrease the number of total sets according to the patient´s toleranceWhen patient completed strength training exercise >15 repetitions:1) Increase the load until patients reach `momentary muscular failure´ between 8 to 15 repetitions2) Increase the number of total sets according to the patient´s tolerance |
Abbreviations: ACCP – American College of Chest Physicians, AACVPR – American Association of Cardiovascular and Pulmonary Rehabilitation, BTS – British Thoracic Society, ATS – American Thoracic Society, ERS – European Respiratory Society, RM – repetition maximum, RCT – randomized controlled trial.
We initially developed a 64-item online survey to understand international expert practices on the delivery of exercise training in the setting of PR in patients with CRDs like chronic obstructive pulmonary disease, asthma, or interstitial lung disease. Peers checking for plausibility and consistency proofread the survey before its dissemination. The survey consisted of mixed open-ended questions and multiple-choice questions. Most of the questions afforded multiple answers – no question was mandatory to be addressed. The survey was built by using SurveyMonkey Software.
Dissemination of the surveyThe survey was sent by email to 45 international experts from the field of exercise training in CRD patients and was available from 18 January 2022 to 18 February 2022. Participants for the survey were selected from contributor lists of previous international surveys and statements on PR5,9 and were partly expanded by experts based on the experiences and judgment of the authors. Only one expert per center was invited to participate in the survey to avoid a center-based bias. Participation in the survey was voluntary and participants were asked if they agreed that their names would be disclosed in the section of acknowledgments.
Data analysisQuantitative data were reported as percentages of answers for each question of the survey.
ResultsThirty-five out of 45 invited experts from 21 countries across 5 continents (e-Figure 1) completed the survey (response rate: 78%). The professional background of the experts was multidisciplinary, consisting of physiotherapists (66%), pulmonologists (20%), and clinical exercise physiologists (14%). Most experts provide outpatient programs (71%) of 8 to 12 weeks, including 2 to 3 training sessions per week or inpatient exercise programs (37%) for 3 to 4 weeks applying 5-7 exercise sessions per week (e-Figures 2-4). All experts (100%) provide endurance training and 94% apply strength training. The online supplement includes all survey questions and the experts´ responses.
Tables 1-2 summarise the findings from the survey relevant to CET, IET and ST recommendations reported within international respiratory society statements, and guidelines as well as evidence from the literature.
Endurance trainingCycling (97%) and walking (ground floor 86% or treadmill 77%) were the most common endurance training modalities, and continuous endurance training (CET, 83%) or interval endurance training (IET, 86%) were the most used modes (e-Figures 8-9). Criteria to prescribe IET instead of CET were: when patients do not tolerate CET due to dyspnoea at the initial training session (79%), intense breathlessness during the initial exercise assessment (76%), or profound exercise-induced oxygen desaturation (59%). The following measurements are usually performed during CET and IET: 10-point Borg scale rating of breathlessness (94%) and leg discomfort (86%), oxygen saturation (87%), and heart rate (80%) (e-Figures 20 and 33).
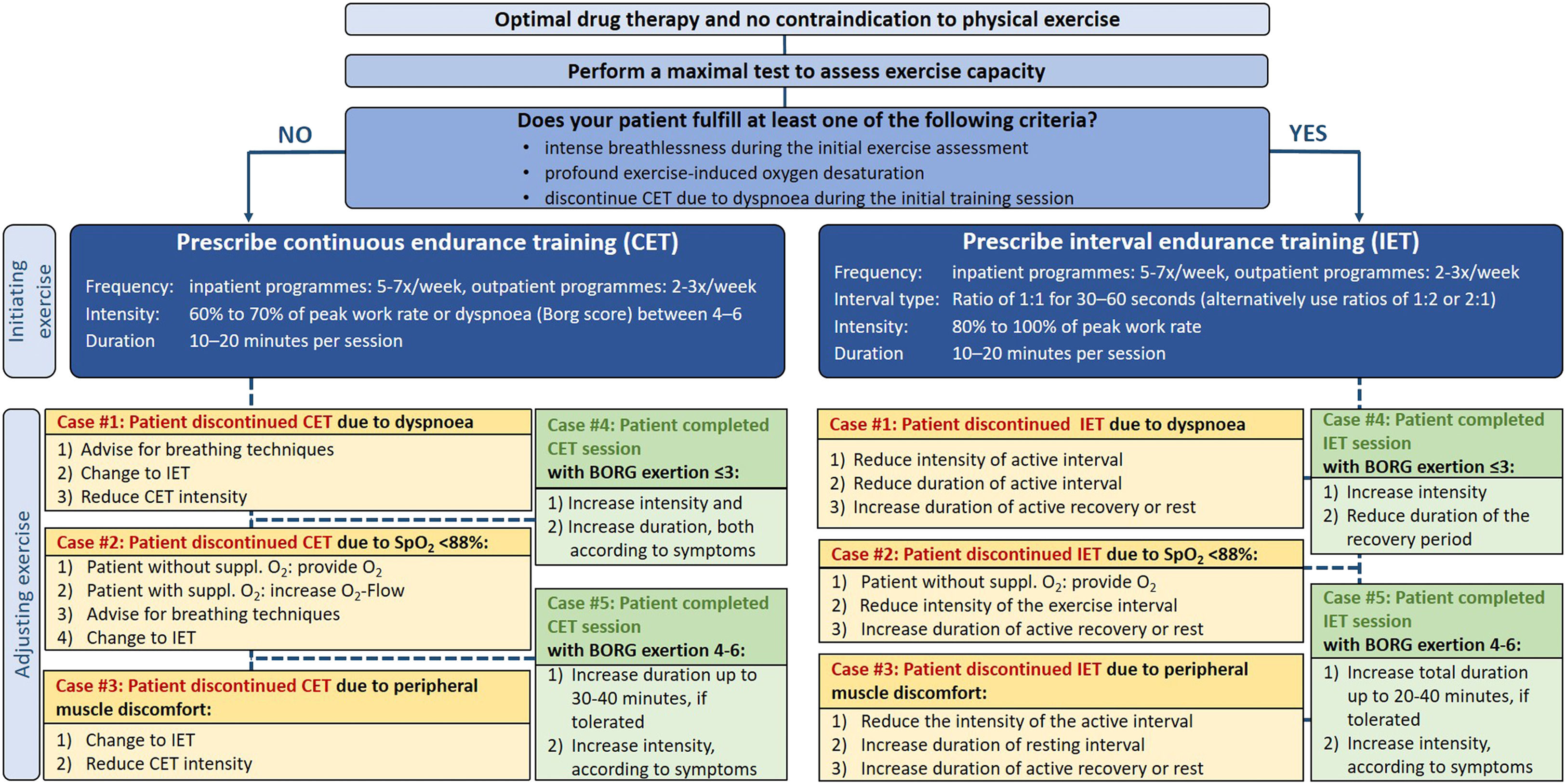
Setting initial CET loadTo set initial training intensity, 82% of experts start CET at 60-70% of peak work rate and 77% use the 10-point Borg scale to set exercise intensity aiming for a dyspnoea score between 4 and 6. Seventy-four percent prescribe a total duration of 10 to 20 minutes during the first training session (Table 1).
Adjusting CET load during an ongoing training programTo advance the training load, 57% of experts initially increase the duration of exercise, aiming for a total CET duration of 30 to 40 minutes per session (71% of experts, e-Figure 21). As a second step, the intensity is increased. The rate of intensity progression is variable, as 71% increase the intensity depending on the patients´ symptoms (breathlessness and/or leg discomfort, e-Figure 25). Fig. 1 provides an overview on experts´ practices, when patients need to interrupt a CET session due to various reasons.
Setting initial IET loadMost experts (59%) use a ratio of 1 to 1 alternating exercise with active or complete recovery periods. Work to recovery ratios of 1 to 2 (29%) or 2 to 1 (26%) are also used (e-Figure 35). Periods of 30 to 60 seconds are usually (69%) implemented as the length of time for the interval (exercise or recovery) phases (e-Figure 36). The initial intensity for the work interval is mostly (78%) set between 80% to 100% of baseline peak work rate with an initial total exercise duration of 10 to 20 minutes (61%) during the first training session (e-Figures 37 and 41).
Adjusting IET load during an ongoing training programAll experts (100%) agreed that it is necessary to progressively adjust the load during IET (e-Figure 43) with 76% allowing a total IET duration ranging between 20 and 40 minutes per session based on the patients´ tolerance (e-Figure 42). Also, 76% of experts progressively increase the intensity of exercise during IET based on the patients´ symptoms (e-Figure 45). Fig. 1 provides an overview on experts´ practices when patients discontinue a IET session due to various reasons.
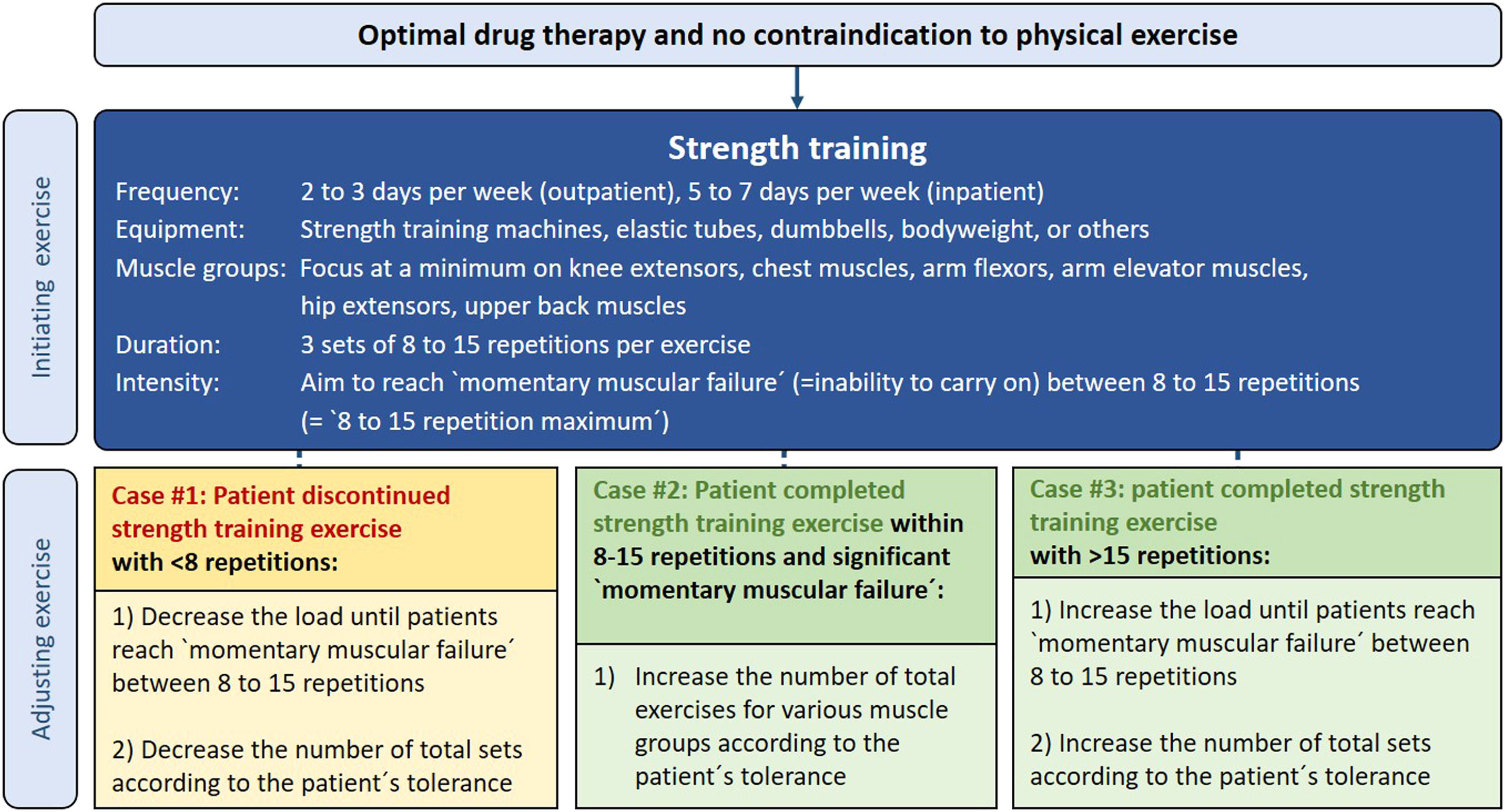
Strength training (ST)Experts used various ST apparatus, such as regular weight training machines (79%), elastic tubes (56%), dumbbells (53%), or bodyweight only (53%) (e-Figure 52). The following muscle groups were seen as a minimum standard for ST: knee extensors (91%), chest muscles (59%), arm flexors (59%), arm elevator muscles (56%), hip extensors (56%), and upper back muscles (50%) (e-Figure 53). Fifty-one percent of experts do not perform a 1-repetition maximum test at the beginning of a ST program (e-Figure 54).
Setting initial load for STMost experts (68%) perform 3 sets per exercise (e-Figure 58). 62% of experts set the intensity at a specific load that patients can tolerate for a range of 8 to 15 repetitions per set, whereas 56% of experts use a range of around 60% to 70% from the 1-repetition maximum test to determine the initial ST intensity (e-Figure 55). Also, 56% of experts advise patients to reach local muscular exhaustion (or close to it) at the end of a single ST set (e-Figure 59).
Adjusting ST load during an ongoing training programWhen patients can complete all sets during an exercise workout without significant exhaustion, experts increase the load to the next higher tolerated workload (41%), increase the weight by 5 to 10% of the 1-repetition maximum (35%) or increase the training load until patients reach local muscular exhaustion (or close to it) at the end of a single set (35%) (e-Figure 63). 53% of experts also extend the total number of exercises, according to the patients´ tolerance to further progress training volume (e-Figure 64).
Exercise prescription flowchartsExercise flowcharts were developed (Figs. 1 and 2) to facilitate tailoring of exercise training prescription to the individual patient's needs and capabilities. The expert practices on adjusting exercise training for the presented cases, were derived on the basis of the most prevalent responses to the survey questions (see online supplement).
DiscussionWe conducted a survey to capture the practices of international experts in delivering exercise training for patients with CRDs. Based on the expert clinical practice, exercise flowcharts were developed including suggestions on prescribing and adjusting continuous, interval, and strength training in patients with CRDs. International experts from 35 different centres across 21 countries completed the survey. This reflects the largest collection of multiple expertise within this field and provides an update and extension of previously published practical recommendations on exercise training in patients with COPD.13 Interestingly, there was considerable heterogeneity amongst experts on how they apply and adjust exercise training. This shows that there are several ways to reach the same aim for improving physical performance.
International guidelines and statements on PR provide recommendations on how exercise training in patients with CRDs should be prescribed (Tables 1–2). Our survey indicates that exercise training practices closely align with these guidelines. However, guidance on how to further adjust exercise training workload during an ongoing exercise training program and how to deal with situations when patients need to discontinue a session of exercise training due to various limitations, is scarce. Former surveys about exercise prescription also focussed more on exercise programme structures and modalities rather than on practical advice on tailoring exercise training workload.14,15 However, these aspects are highly relevant to the delivery of exercise training, and as such our flowcharts may provide further practical guidance on prescribing and adjusting exercise training according to the individual patient's needs and capabilities.
Endurance training (CET or IET) is consistently considered a fundamental component of exercise training in patients with CRDs.5,16-18 Most endurance training programmes are based on the CET method, in which exercise is performed at a constant intensity for an extended period without interruption. However, patients with severe CRDs are usually unable to sustain CET at relatively high intensities for an extended period due to increased respiratory distress.19,20 IET, which consists of repeated bouts of maximal/high-intensity exercise, alternated with short intervals of rest or low-intensity exercise, is a suitable alternative to CET.21 Several systematic reviews have consistently concluded that CET and IET demonstrate comparable efficacy in improving exercise capacity, exercise-induced dyspnoea sensations, muscle fiber structure and function, and quality of life.10,22 However, there is also some evidence, that especially patients with advanced CRDs perceive less dyspnoea during IET compared to CET.20,23 Underlying physiological reasons include a lower reliance on anaerobic glycolysis associated with lower ventilatory requirement and degrees of dynamic hyperinflation during IET.24,25 This explains the greater tolerance to IET compared to CET in patients with advanced CRDs. In our flowchart we presented practical indications from the experts´ experiences on when to consider IET instead of CET. Furthermore, practical cases are presented, that show how experts proceed, when patients need to interrupt CET or IET due to various reasons.
Studies have shown, that endurance training combined with strength training results in significantly greater improvements in muscle strength, muscle hypertrophy, and quality of life compared to endurance training alone in patients with COPD.11,12,26,27 Therefore, when patients with COPD have the capacity to perform a combined endurance and strength training program, this may provide the optimal exercise prescription.
One of the most important considerations concerning strength training is intensity. The general use of the term `intensity´ in the strength training literature, including the ACSM statement,28 refers to the load used (i.e. the fraction of 1 repetition maximum, %1RM). However, the expression of %1RM only represents the training load given as a fraction of maximal effort. The %1RM does not explicitly imply how hard an individual is working during a set of strength exercises. Therefore, Fisher and colleagues proposed that `intensity´ in its truest sense is the level of a subject´s effort applied to a given load.29 The inability to perform additional concentric contractions at a given load without significant changes in posture or movement speed was defined as `momentary muscular failure´.29 It has been shown that training to `momentary muscular failure´, maximizes muscle fibre recruitment (especially to the higher threshold fast-twitch muscle fibres), and increases the secretion of growth-promoting hormones compared to not reaching `momentary muscular failure´.30 Both are important factors for the capability of producing the greatest increases in muscle strength and hypertrophy.
To date, many studies have investigated the benefits of strength training and the different approaches to determine the optimal strength training intensity/load. Several systematic reviews have consistently concluded that muscle hypertrophy can be equally achieved across a wide spectrum of loads (30%1RM to 90%1RM).29,31,32 This evidence suggests that reaching `momentary muscular failure´ at the end of a strength training set (i.e. within a range of 8 to 15 repetitions) is the most important aspect for maximizing muscle hypertrophy. Therefore, it is not necessary to perform a 1RM effort for prescribing strength training intensity at a certain fraction of 1RM, because the fraction of 1RM alone is not relevant for improving muscle hypertrophy.29 Instead, it is the effort that a subject perceives as strenuous.
We are aware that the requirements for exercise training programmes in patients with CRDs vary widely across the world within different healthcare systems and local infrastructure. However, different types of exercise training apparatus (e.g. regular strength training machines, free weights, elastic tubes, etc.) can potentially increase exercise performance, when appropriate exercise training principles and intensity progression to reach `momentary muscular failure´ are applied.33,34 Recent studies have shown that an exercise training program using minimal equipment can be equally effective in increasing muscle strength and exercise performance compared to using sophisticated exercise apparatus.33,34 Therefore, what is much more relevant is how adequately patients exercise and not what kind of apparatus they use. Hence, our flowchart suggestions are also transferable to different exercise training settings.
Some limitations need also to be addressed. First, there is no direct proof of the benefits of the training recommendations included in our flowcharts. A clinical validation is needed. However, we suggest that the meaningful benefits shown in numerous exercise training studies might also apply to our expert-based recommendations since there is a substantial overlap in several basic training principles between the experts´ practices and scientific evidence.
Second, there was no official panel discussion involving all experts like a Delphi consensus process. Third, we did not ask for safety issues related to the experts´ experiences with exercise training. However, exercise training is usually accepted as a safe intervention for patients with CRDs following a baseline patient assessment to rule out any contraindications for physical exercise.5 Fourth, we provided general recommendations for a range of CRDs and did not take disease-specific considerations into account (i.e. an interval warm-up phase of 10-15 minutes in patients with asthma to prevent exercise-induced bronchoconstriction during a subsequent endurance training).35
Finally, the current flowcharts do not take into account non-invasive ventilation during exercise training,36 or other types of lower-limb muscle training, such as neuromuscular electrical stimulation,37 single-leg cycling,38 and whole-body vibration.39
A strength of this survey is the large collection and consolidation of international experts´ experiences for adjusting exercise training in patients with CRDs during an ongoing training program.
During the process of this survey, we have also identified important questions for future research like what patient/modality combinations are the best to safely achieve the largest training effects? What are the underlying changes in exercise performance, including intramuscular changes40 and oxygen uptake kinetics.41 Furthermore, is there a valid approach to optimise walking speed during ground-based walking training? Future studies should include a larger number of rehabilitation experts and should collect more data with a more detailed description of the expert responses, in order to standardize procedures at a world level in the area of exercise training in CRDs.
In conclusion, based on these experts´ experiences, exercise prescription flowcharts were developed. These flowcharts are meant to guide healthcare professionals in prescribing and adjusting continuous, interval, and strength training in patients with chronic respiratory diseases.
AcknowledgmentsGuarantorRG had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis.
Other contributionsThe authors thank all the participants who completed the survey, allowing the high response rate. We thank the participants for sharing their knowledge and experiences, which may support healthcare professionals worldwide by providing exercise training to patients with chronic respiratory diseases. The following experts answered the survey and agreed to be named here (in alphabetical country order):
Christian Osadnik (Melbourne, Australia), Vinicius Cavalheri (Perth, Australia), Ralf Zwick (Vienna, Austria), Chris Burtin (Hasselt, Belgium), Daniel Langer (Leuven, Belgium), Heleen Demeyer (Ghent, Belgium), Carlos A. Camillo (Londrina, Brazil), Alberto Neder (Kingston, Canada), Linette Kofod (Hvidovre, Denmark), Isabelle Vivodtzev (Paris, France), Rainer Gloeckl (Schönau am Königssee, Germany), Janos Varga (Budapest, Hungary), Stefano Belli (Veruno, Italy), Guido Vagheggini (Volterra, Italy), Akita Tamaki (Kobe, Japan), Atsuyoshi Kawagoshi (Akita, Japan), Enock Chisati (Blantyre, Malawi), Steffi Lemmens-Janssen (Basalt, Netherlands), Maurice Sillen (Horn, Netherlands), Anita Grongstad (Jessheim, Norway), Bente Frisk (Bergen, Norway), Alda Marques (Aveiro, Portugal), Catarina Santos (Lisbon, Portugal), Elena Gimeno-Santos (Barcelona, Spain), Karin Wadell (Umea, Sweden), Gilbert Büsching (Barmelweid, Switzerland), Thomas Riegler (Heiligenschwendi, Switzerland), Spencer Rezek (Winterthur, Switzerland), Melda Saglam (Ankara, Turkey), Ioannis Vogiatzis (Newcastle, UK), Rachael Evans (Leicester, UK), Don S Urquhart (Edinburgh, UK), Rebecca Crouch (Durham, USA)
Financial/nonfinancial disclosuresAll authors declare that they have no conflicts of interest.
Role of sponsorsThis study did not receive any funding.
Funding informationThis study did not receive any funding.