Pneumonia is a leading cause of childhood mortality globally. Children with severe pneumonia associated with hypoxaemia require oxygen (O2) therapy, which is scarce across resource-constrained countries. Solar-powered oxygen (SPO2) is a novel technology developed for delivering therapeutic O2 in resource-constrained environments.
Research questionIs the introduction of SPO2 associated with a reduction in mortality, relative to the existing practice?
Study designThis was a pragmatic, quasi-experimental study comparing mortality amongst children < 5 years of age with hypoxaemic respiratory illness before and after the installation of SPO2 in two resource-constrained hospitals.
MethodsParticipants were children < 5 years old admitted with acute hypoxaemic respiratory illness. The intervention was SPO2, installed at two resource-constrained hospitals. The primary outcome was 30-day mortality. Secondary outcomes included in-hospital mortality (time to death), length of hospital stay among survivors, duration of O2 therapy (time to wean O2), and O2 delivery system failure(s).
ResultsMortality amongst children admitted with acute hypoxaemic respiratory illness decreased from 30/50 (60%) pre-SPO2 to 15/50 (30%) post-SPO2 (relative risk reduction 50%, 95%CI 19 – 69, p = 0.0049). The post-SPO2 period was consistently associated with decreased mortality in statistical models adjusting for potential confounding factors. Likewise, survival curves pre- and post- SPO2 differed significantly (hazard ratio 0.39, 95% CI 0.20 – 0.74, p = 0.0043). A reduction in the frequency of O2 delivery interruptions due to fuel shortages and multiple patients needing the concentrator at once was observed, explaining the mortality reduction.
InterpretationSolar-powered oxygen installation was associated with decreased mortality in resource-constrained settings.
Pneumonia is a leading cause of mortality among children under 5 years of age, accounting for more than 800,000 deaths annually.1 Oxygen (O2) is an essential therapy for hypoxaemic illnesses, including pneumonia; however, O2 is often not available on paediatric wards in resource-constrained hospitals.2,3 In the current COVID-19 pandemic, O2 demand is expected to increase dramatically in low- and middle-income countries, exacerbating this pre-existing shortage.
In resource-constrained settings, methods of delivering O2 include O2 cylinders and grid-powered O2 concentrators,4,5 both of which are limited by cost and logistical issues.2,6 Studies conducted in Uganda and Kenya reported that < 20% of paediatric wards in district hospitals have access to functional O2 delivery systems,2 with facilities experiencing power outages 7% of the time.7
Improved O2 delivery systems can lead to significant improvements in mortality from childhood pneumonia.8 We have previously detailed the design and implementation of a novel method of O2 delivery capable of implementation in remote locations with limited access to consistent electrical supplies: solar powered oxygen (SPO2).9,10 Solar powered O2 involves photovoltaic cells to collect solar energy, which is then stored in a battery bank and used to power an O2 concentrator for the production of medical grade O2. The feasibility, safety, and efficacy of SPO2 has been demonstrated through a proof-of-concept study and a randomized controlled trial, showing non-inferiority compared to cylinder O2 in terms of hospital length of stay, duration of O2 therapy, and recovery time.9,10 An evaluation of the impact of SPO2 on mortality is warranted.
The objective of this study was to compare the mortality among infants and children admitted with acute hypoxaemic respiratory illness at two resource-constrained hospitals in the Democratic Republic of the Congo (DRC) before and after installation of SPO2. We hypothesized that the introduction of a reliable source of O2 would be associated with a reduction in mortality, relative to the existing practice, in which the O2 supply was scarce and inconsistent.
MethodsStudy designThis was a pragmatic, quasi-experimental study comparing the mortality amongst children under 5 years of age admitted with acute hypoxaemic respiratory illness before (pre-SPO2) and after (post-SPO2) the installation of SPO2 in two resource-constrained hospitals. Before-after designs have been used in several recent trials evaluating O2 systems in resource-constrained settings,11 novel O2 delivery methods,12 other respiratory therapies,13-15 and childhood infections.16,17 Although subject to limitations such as temporal trends, before-after study designs are effective research tools that, in some cases, have changed practice.18 To mitigate potential biases inherent in this study design, we used prospective data collection with consistent reporting criteria and multivariable statistical methods to correct for potential confounding factors.
SettingThe DRC ranks 179th out of 188 countries in terms of the human development index and 88% of the population lives on less than US $1.25 per day.19 The mortality under five years of age is 300,000/year, with 45,000 deaths/year due to pneumonia.20 The delivery of health services is complicated by ongoing armed conflicts and security concerns, which contribute to the elevated mortality rates in these regions.21 The DRC has also experienced outbreaks of Ebola virus, with the most recent outbreak in North Kivu beginning in August 2018, during this study's implementation. Availability of O2 remains poor across rural parts of the DRC.22
The study enrolled hypoxaemic children admitted to two hospitals in Butembo, DRC. Matanda Hospital is a 260-bed facility, with a 6-bed intensive care unit. The solar powered O2 concentrator was installed in the intensive care unit during the present study. The Centre Hospitalier Universitaire du Graben is a 178-bed facility, with dedicated pediatric and neonatal wards. The solar powered O2 concentrator was installed in the neonatal ward during the present study. Of note, the solar powered O2 concentrators were not present during the pre-SPO2 period at either site. The two hospitals have a high ratio of patients to healthcare providers (approximately one nurse for every 20 patients). Each site was staffed by one general practitioner and one pediatrician. Fingertip pulse oximeters (ChoiceMMmed brand, 2018Beijing Choice Electronic Tech Co., Beijing, China) were used for spot checks of O2 saturation. Use of pulse oximetry was individualized and guided by the individual clinicians, according to clinical judgement.
ParticipantsPatients presenting to selected sites meeting the following criteria were included: (1) age < 5 years; (2) hypoxaemia (O2 saturation < 90%); (3) warranted hospital admission based on clinical judgement.
Intervention and Study proceduresThe solar powered O2 system has been previously described.10 In brief, the system consisted of locally sourced solar panels, charge controller, battery bank, DC/AC current inverter, and a 300 W O2 concentrator (model 525 KS, DeVilbiss, Healthcare LLC, Somerset, PA, USA).10 The system components were purchased from and installed by a Congolese non-profit association providing essential medicines and equipment throughout the DRC (Association Régionale D'Approvisionnement en Médicaments Essentiels, ASRAMES, Goma, DRC).
Eligible participants’ parents or legal guardians were approached for written informed consent to participate in the study. The study's purpose, benefits, risks, confidentiality, and alternatives were explained to parents or legal guardians in the appropriate language. Patients received standard care for their underlying illness. Fifty patients were recruited in both in the pre- and post- implementation periods, a sample size similar to previous before-after designs in the field.12
Demographic information and clinical data were collected from the medical records. After discharge, follow-up was done by telephone to determine vital status 30 days after admission (primary outcome). The need for O2 therapy was evaluated daily using standard operating procedures for weaning O2. The final disposition of the patients was recorded (discharged with or without disability, transferred to another facility, absconded, death), and the length of stay was calculated amongst survivors.
Outcome measuresOur primary endpoint was mortality at 30 days post-admission. Secondary outcomes were in-hospital mortality (time to death), length of hospital stay among survivors, duration of O2 therapy (time to wean O2), and O2 delivery system failure(s).
Statistical considerationsDescriptive statistics used number and percentage for binary variables and median with interquartile range for continuous variables. Comparative statistics used chi-squared or Fisher's exact test, as appropriate, for binary variables and Mann-Whitney U-test for continuous variables. Kaplan-Meier survival analysis was used to compare the time to death pre- and post-SPO2. We used stratified analysis to examine mortality across strata that could confound the association of SPO2 and mortality. There was no missing data pertaining to the primary outcome. We used logistic regression models to examine the effect of SPO2 on mortality while adjusting for clinical and statistical co-variates (R version 4.0.0, R Computing, Vienna, Austria). Model selection was guided by both biological and statistical considerations. Models were restricted to a maximum of five independent variables for parsimony and to avoid overfitting (limited sample size with 45 deaths).
Ethics approvalThe study was approved by the Comité d’Éthique du Nord Kivu (Centre Hospitalier Universitaire du Graben, Butembo, DRC, Protocol number 005/TEN/2017) and by the Research Ethics Board of the University of Alberta (Study ID Pro00061203).
ResultsFifty patients were recruited between 1 September 2017 and 19 March 2018 (pre-SPO2). Solar-powered oxygen systems were installed in both hospitals from 5-8 October, 2018. Fifty patients were then recruited between 10 October 2018 and 1 August 2019 (post-SPO2). Table 1 shows the demographic and clinical features of the pre-SPO2 and post-SPO2 groups. Most differences between the pre-SPO2 and post-SPO2 groups were not statistically significant, with key data presented in Table 1. Table 2 shows the treatment and outcome for patients enrolled pre- and post-SPO2.
Characteristics at admission of 100 children under 5 years of age hospitalized with hypoxaemia.
| Overall (N = 100) | Pre-SPO2 (N = 50) | Post-SPO2 (N = 50) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age [months] median (IQR) | 2 (0-8.2) | 2.2 (0-10) | 2 (0-4.8) | 0.18 |
| <2 months | 42 (42) | 19 (38) | 23 (46) | 0.54 |
| 2-59 months | 58 (58) | 31 (62) | 27 (54) | |
| Female sex | 48 (48) | 22 (44) | 26 (52) | 0.55 |
| Site | 0.0088 | |||
| CHU Graben | 44 (44) | 15 (30) | 29 (58) | |
| Matanda Hospital | 55 (55) | 35 (70) | 21 (42) | |
| Clinical features | ||||
| Cough | 72 (72) | 26 (52) | 46 (92) | <0.0001 |
| Fever prior to admission | 68 (68) | 29 (58) | 39 (78) | 0.054 |
| Unable to feed or drink | 70 (70) | 36 (72) | 34 (68) | 0.83 |
| Vomiting everything | 10 (10) | 6 (12) | 4 (8) | 0.74 |
| Lethargic | 79 (79) | 40 (80) | 39 (78) | >0.99 |
| Convulsions | 28 (28) | 14 (28) | 14 (28) | >0.99 |
| Physical findings | ||||
| Severely underweight1 | 33 (33) | 19 (38) | 14 (28) | 0.39 |
| Mid-upper arm circumference [mm], median (IQR) | 110 (100-130) | 110 (100-130) | 120 (100-130) | 0.72 |
| SpO2 [%], median (IQR) | 70 (60-78) | 70 (60-77) | 71 (60-80) | 0.45 |
| Temperature [°C], median (IQR) | 38 (36-38) | 38 (36-38) | 38 (36-38) | 0.63 |
| Tachycardia2 | 24 (24) | 17 (34) | 7 (14) | 0.035 |
| Tachypnea2 | 28 (28) | 12 (24) | 16 (32) | 0.50 |
| Chest indrawing | 80 (80) | 37 (74) | 43 (86) | 0.21 |
| Deep breathing | 69 (69) | 27 (54) | 42 (84) | 0.0025 |
| Composite clinical severity score | ||||
| RISC, median (IQR)3 | 6 (5-6) | 6 (5-6) | 6 (5-6) | 0.94 |
| Laboratory | ||||
| Haemoglobin [g/L], median (IQR)4 | 110 (90-130) | 110 (90-120) | 110 (90-130) | 0.38 |
| Glucose [mmol/L], median (IQR)5 | 4.4 (3-7.2) | 6.7 (3.4-9.9) | 3.2 (3-4.5) | 0.037 |
| Primary diagnosis | ||||
| Pneumonia | 33 (66) | 16 (32) | 17 (34) | >0.99 |
| Sepsis | 29 (29) | 18 (36) | 11 (22) | 0.19 |
| Malaria | 20 (20) | 5 (10) | 15 (30) | 0.023 |
| Neonatal respiratory distress syndrome | 9 (18) | 4 (8) | 5 (10) | >0.99 |
| Bronchiolitis | 3 (3) | 2 (4) | 1 (2) | >0.99 |
| Congenital heart disease | 3 (3) | 3 (6) | 0 (0) | 0.24 |
| Ebola virus disease | 1 (1) | 0 (0) | 1 (2) | >0.99 |
| Other6 | 2 (2) | 2 (4) | 0 | >0.99 |
Values represent n (%) unless otherwise specified
Clinical management and outcomes of 100 children under 5 years of age hospitalized with hypoxaemia.
| Overall (N = 100) | Pre-SPO2 (N = 50) | Post-SPO2 (N = 50) | p-Value | |
|---|---|---|---|---|
| Oxygen treatment | ||||
| Duration of O2 therapy [hours], median (IQR)1 | 16 (9.7-48) | 6.5 (4.0-24) | 20 (12-57) | 0.0049 |
| Total volume of O2 delivered [× 1000 L], median (IQR)1 | 39 (22-110) | 15 (9.0-68) | 50 (31-120) | 0.0049 |
| SpO2 at last encounter [%], median (IQR) | 91 (52-95) | 81 (55-96) | 94 (42-95) | 0.56 |
| Interruptions to O2 therapy | 27 (27) | 26 (52) | 1 (2) | <0.0001 |
| Fuel shortages | 12 (12) | 12 (24) | 0 (0) | 0.00023 |
| Multiple patients | 15 (15) | 14 (28) | 1 (2) | 0.00039 |
| Antibiotic Use | ||||
| Ampicillin | 9 (9) | 8 (16) | 1 (2) | 0.031 |
| Gentamicin | 59 (59) | 29 (58) | 30 (60) | >0.99 |
| Cefotaxime or Ceftriaxone2 | 93 (93) | 43 (86) | 50 (100) | 0.012 |
| Metronidazole | 10 (10) | 9 (18) | 1 (2) | 0.016 |
| Other3 | 9 (9) | 7 (14) | 2 (4) | 0.16 |
| Other Treatments | ||||
| Intravenous glucose | 73 (73) | 26 (52) | 47 (94) | <0.0001 |
| Antipyretics | 60 (60) | 24 (48) | 36 (72) | 0.025 |
| Length of Stay, median (IQR)1 | 7 (5-9) | 6 (5-7) | 8 (5-10) | 0.054 |
| Mortality | ||||
| At 48 hours after admission | 30 (30) | 18 (36) | 12 (24) | 0.28 |
| In hospital | 43 (43) | 30 (60) | 13 (26) | 0.0012 |
| At follow-up 30 days after admission | 45 (45) | 30 (60) | 15 (30) | 0.0049 |
The 30-day mortality among hypoxaemic infants and children was 30/50 (60%) pre-SPO2 and 15/50 (30%) post- SPO2 (p = 0.0049). This represents a relative risk reduction of 50% (95% CI 19 – 69%) and a number needed to treat of 3.3 (95% CI 2.1 – 8.8). The survival curves pre- and post-SPO2 differed significantly (hazard ratio 0.39 [95%CI 0.20-0.74], p = 0.0043, Fig. 1).
There was a significant reduction in the number of patients experiencing interruptions to their O2 therapy from 26/50 (52%) pre-SPO2 and 1/50 (2%) post-SPO2 (p < 0.0001). More specifically, there were fewer interruptions due to fuel shortages (12/50 [24%] to 0/50 [0%], p = 0.00023) and decreased need to share O2 therapy among multiple patients (14/50 [28%] to 1/50 [2%], p = 0.00039). The duration of O2 therapy and the total volume of O2 administered increased significantly from pre-SPO2 to post-SPO2 (Table 2)
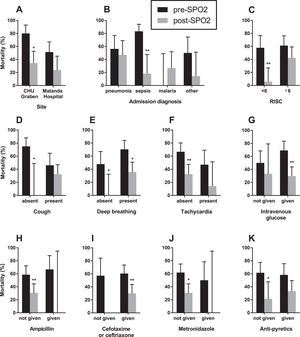
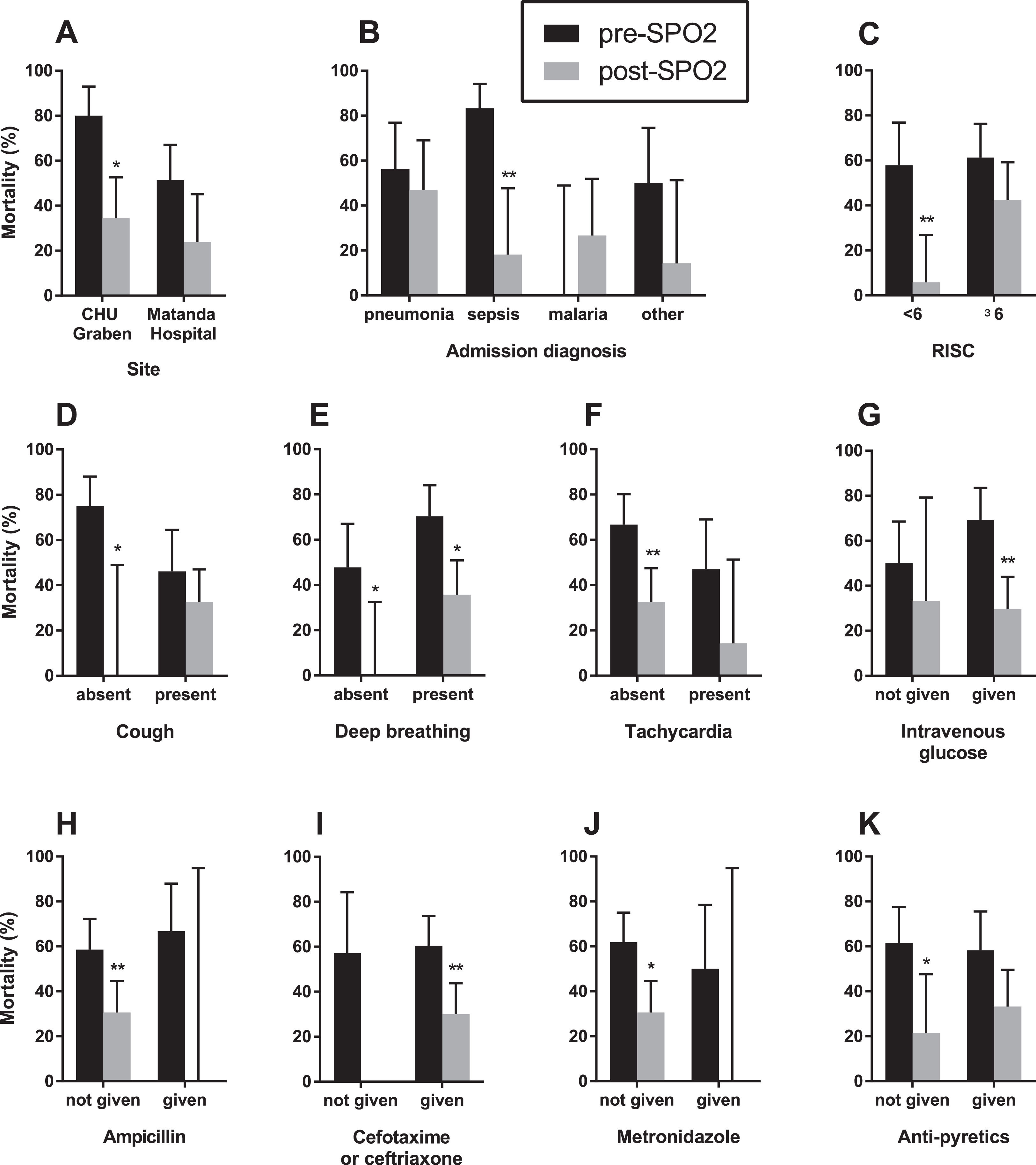
Because of the quasi-experimental design, several potential confounding factors were identified to be different in the pre-SPO2 and post-SPO2 periods including site, markers of disease severity (presence of cough, tachycardia, deep breathing), primary diagnosis (malaria versus pneumonia, sepsis and other conditions), and co-treatments (intravenous glucose, third-generation cephalosporin, ampicillin, metronidazole, and antipyretics) (Table 2). We performed a stratified analysis, examining the difference in mortality pre- and post- SPO2 in these subgroups (Fig. 2). We found a consistent reduction in mortality across multiple subgroups, suggesting that there was no confounding that would have affected the observed mortality difference pre- and post-SPO2.
Stratified analysis of mortality differences amongst 100 infants and children hospitalized with hypoxaemia pre- and post-SP02 installation. Across all strata, there was a decrease in mortality in post-SPO2 compared to pre-SPO2. * - significantly different (p < 0.05); ** - significantly different (p < 0.01).
We next constructed multivariable logistic regression models to adjust for potential effects of co-variates (Supplemental e-Table 1). Model 1 adjusted for disease severity (RISC, coded as a continuous variable) and diagnosis (categorical variable with four levels, pneumonia, sepsis, malaria, and other), and showed that the association between lower mortality post-SPO2 remained statistically significant (adjusted odds ratio 0.31 [95%CI 0.12-0.77], p = 0.013). Model 2 adjusted for potential factors affecting mortality based on statistical considerations. Cough and malaria were both statistically significantly associated with reduced mortality and were more frequent post-SPO2. In addition, although not statistically significant, treatment with cefotaxime/ceftriaxone and intravenous glucose were associated with reduced mortality and were more frequent post-SPO2. Model 2, adjusting for cough, malaria, cefotaxime/ceftriaxone, and intravenous glucose treatment, showed that the association between lower mortality post-SPO2 remained statistically significant (adjusted odds ratio 0.30 [95%CI 0.095-0.89], p = 0.033). In summary, the post-SPO2 period was consistently and robustly associated with decreased mortality in statistical models adjusting for potential factors associated with mortality that were different pre- and post-SPO2 (Supplemental e-Table 2).
DiscussionThis study found that implementation of SPO2 systems was associated with a 50% decrease in 30-day mortality in children with hypoxaemic respiratory illness. Installation of SPO2 systems was associated with a reduced frequency of interruptions to O2 therapy due to fuel shortages or need to share systems among multiple patients, suggesting an improvement in the quality of care being delivered.
Oxygen availability remains a challenge in many resource-constrain settings around the world. In a survey conducted in Kenya, approximately 20% of rural healthcare facilities lacked O2 equipment. Of those with equipment, a third of their patients faced interruptions lasting a median of 11 minutes, with less than 20% having access to backup O2 cylinders.7 In Uganda, only 18% of paediatric wards had access to functional O2 delivery systems.2 Similar shortages have been reported in other resource-constrained settings across Africa.5,23 Similarly, in the current study, prior to SPO2 installation, 52% of patients experienced interruptions to their O2 therapy, half of which were due to fuel shortages leading to concentrator failure. After installing SPO2, only a single patient (2%) experienced an interruption to their O2 therapy, in their case due to multiple paediatric patients requiring the O2 concentrator simultaneously. Among survivors, the duration of O2 therapy and the total volume of O2 delivered were statistically significantly greater after SPO2 installation (Table 2), reflecting fewer interruptions and greater O2 availability. Overall, these findings suggest that the lack of available O2 equipment together with frequent power interruptions contribute to the elevated mortality associated with hypoxaemic respiratory illnesses.
While hypoxaemia is known to be associated with an increased risk of mortality in patients with acute respiratory illnesses, the mortality rate seen in our study before the implementation of SPO2 (60%) was higher than many past reports.24 This may reflect the remote, rural, and severely resource-constrained hospitals selected for this study (e.g., lack of reliable access to O2 as well as ventilators). Danger signs (i.e., not able to drink, persistent vomiting, convulsions, lethargy, stridor at rest, or severe malnutrition)25 were common in our cohort and were associated with subsequent mortality (Supplemental e-Table 1), consistent with previous studies. Of note, the frequency of danger signs and the RISC scores did not differ between the pre- and post-SPO2 periods, suggesting that the severity of presenting illness was comparable pre- and post-SPO2.
There were a number of differences, besides mortality, in patients enrolled in the post-SPO2 period, relative to the pre-SPO2 period: higher proportion of patients from the CHU Graben; higher frequency of cough and deep breathing; lower frequency of tachycardia; and higher proportion diagnosed with malaria (Table 1). Of these factors, only the presence of cough and a diagnosis of malaria were associated with decreased 30-day mortality (Supplemental e-Table 1). In a multivariable logistic regression model including these variables (Model 2, Supplemental e-Table 2), the association between SPO2 and decreased mortality remained statistically significant, suggesting that the higher proportion of children with cough and malaria post-SPO2 did not explain the observed mortality reduction.
This study builds on our group's previous work evaluating SPO2 in resource-constrained settings. Previously, we have demonstrated proof-of-concept,10 non-inferiority relative to cylinder O2,9 and cost-effectiveness26 of SPO2. The current study provides quasi-experimental evidence of mortality reduction associated with SPO2. Additional experimental evidence from cluster-randomized controlled trials will be needed to conclusively show that SPO2 reduces mortality. Currently, we are conducting a stepped-wedge cluster-randomized controlled trial of SPO2 at 20 sites across Uganda.27
The median length of hospital stay was 6 days (interquartile range [IQR] 5-7) pre-SPO2 and 8 days (IQR 5-10) post-SPO2, a difference that approached statistical significance (p = 0.054). The possible prolongation of duration of admission may be due to delay in discharge with improved recognition of clinically apparent hypoxaemia that resulted from increased use of pulse oximeters with our study. Of note, one previous randomized controlled trial demonstrated that SPO2 was not associated with prolonged length of stay, relative to cylinder O2.9
Our study has several limitations. Quasi-experimental before-after designs are subject to the confounding effects of time and lack a distinct control group.18 Indeed, imbalance in co-treatments (e.g., intravenous glucose) pre- and post-SPO2 were directly attributable to improvements in patient care that arose as a result of our study. Potential confounding variables were examined through stratified analyses (Fig. 2) and multivariable modelling (Supplemental e-Table 2). Nonetheless, further investigation is required to conclusively show mortality benefit of SPO2. The limited samples size (50 patients in each group) restricted the number of co-variates that could be included in multivariable models. This sample size is small relative to the burden of disease globally and relative to the patient volumes treated at the participating facilities. A larger study would be desirable to comprehensively adjust for co-variates. Moreover, replication of these findings in other resource-constrained settings would be needed to demonstrate the generalizability of these findings.
ConclusionsSPO2 installation was associated with a marked reduction in mortality among children hospitalized with hypoxaemia in two resource-constrained hospitals. SPO2 is an innovative and simple approach to providing therapeutic O2 to resource-constrained settings. Its ease-of-use, cost-effectiveness, and efficacy in decreasing childhood mortality make it an accessible and effective solution to the high burden of childhood pneumonia. O2 requirements have spiked globally during the ongoing COVID-19 pandemic, and SPO2 can provide a useful countermeasure in resource-constrained settings.
Conflict of interestThe authors have no conflicts of interest to declare.
FundingThis study was funded by The Rotary Foundation (Rotary Global Grant GG1755779). The funder had no role in the study design, analysis and interpretation or results, or decision to publish the manuscript.
Prior Abstract Publication/ PresentationNone.



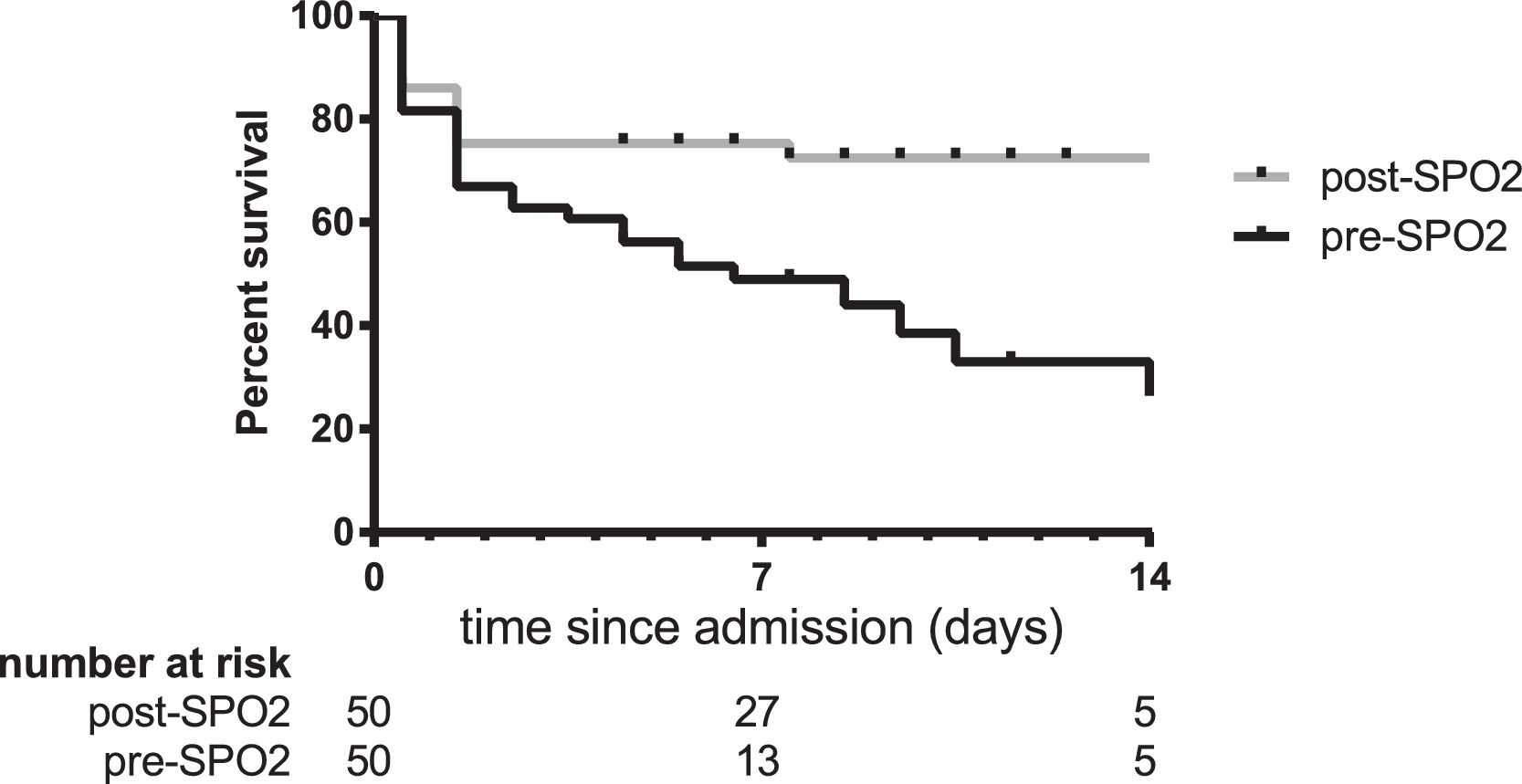
![Survival analysis of 100 infants and children hospitalized with hypoxaemia. The survival curves were significantly different pre- and post-SPO2 implementation (hazard ratio 0.39 [95% CI 0.20-0.74], p = 0.0043). Survival analysis of 100 infants and children hospitalized with hypoxaemia. The survival curves were significantly different pre- and post-SPO2 implementation (hazard ratio 0.39 [95% CI 0.20-0.74], p = 0.0043).](https://static.elsevier.es/multimedia/25310437/0000002900000004/v1_202307031326/S2531043721002245/v1_202307031326/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)