Novel Coronavirus Disease 2019 (Covid-19) is associated with multi-systemic derangement, including circulatory dysfunction with features of endothelial dysfunction, microangiopathic thrombosis and angiocentric inflammation. Recently, intussusceptive angiogenesis has been implicated in the pathogenesis of the disease.
Herein, we conducted a narrative review according to the SANRA guidelines to review and discuss data regarding splitting angiogenesis including mechanisms, drivers, regulators and putative roles. Relevant angiogenic features in Covid-19, including their potential role in inflammation, endothelial dysfunction and permeability, as well as their use as prognostic markers and therapeutic roles are reviewed. Splitting angiogenesis in Covid-19 involve hypoxia, hypoxia-inducible factors, classic angiogenic mediators, such as the Vascular Endothelial Growth Factor (VEGF), Angiopoietins, hyperinflammation and cytokine storm, and dysregulation of the Renin-Angiotensin-Aldosterone System, which combined, interact to promote intussusception.
Data regarding the use of angiogenic mediators as prognostic tools is summarized and suggest that angiopoietins and VEGF are elevated in Covid-19 patients and predictors of adverse outcomes. Finally, we reviewed the scarce data regarding angiogenic mediators as therapeutic targets. These preliminary findings suggest a potential benefit of bevacizumab as an add-on therapy.
Covid-19 is a potentially life-threatening disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection.1 Since its first identification in December 2019, the disease evolved into an international public health emergency, and in March 2020, the World Health Organization declared it a pandemic.
SARS-CoV-2 is associated with a plethora of clinical manifestations ranging from pauci-symptomatic to an invasive, severe infection with acute respiratory distress, systemic hyperinflammation and multi-organ failure.1 SARS-CoV-2 engages the same receptor as SARS-CoV, the angiotensin-converting enzyme 2 (ACE2).2 By expressing abundant ACE2, endothelial cells (EC) are a primordial target to SARS-CoV-2 infection. Accordingly, SARS-CoV-2-mediated endothelial damage is an important issue regarding the virus effects.2-4
MethodsThis narrative review was performed according to the SANRA (Scale for the quality Assessment of Narrative Review Articles) guideline.5 A literature search was performed in PUBMED. The search strategy was focused on articles of four categories: (1) vasculopathic effects of SARS-CoV-2 infection (query used: [Endothelial OR Vasculopathic OR Endothelium] AND [COVID-19 OR SARS-CoV-2]), (2) Vascular effects of cytokine storm (query: [Cytokine OR Cytokine storm] AND [Endothelial OR Vasculopathic OR Endothelium OR Angiogenesis]), (3) Angiogenic features of Covid-19 (query used: [Angiogenesis OR Intussusceptive angiogenesis OR Splitting angiogenesis] AND [COVID-19 OR SARS-COV-2]) and (4) General features of intussusceptive angiogenesis (query used: [Intussusceptive angiogenesis OR Splitting angiogenesis]). The search included articles from a time span ranging 1986 (the year when IA was discovered) and February 2021. Furthermore, we performed an extensive search of the selected articles’ bibliography to retrieve further papers of interest.
Table 1 summarizes the most significant articles evaluated with relevant data.
List of significant papers presenting the most relevant informations.
| Authors | Description | References |
|---|---|---|
| Nishiga M et al | This review summarizes the current understanding regarding the interaction between COVID-19 and the cardiovascular system and its related disorders. | 3 |
| Pons S et al | This article focused on the effects of SARS-CoV-2 infection in the endothelium, describing endothelialitis and endothelial dysfunction that arises in COVID-19. | 4 |
| Iba T et al | This article elucidates the phenomena of COVID-19 associated coagulopathy (CAC), its pathophysiology, prognostic and therapeutic implications. | 10 |
| Patel BV et al | This paper demonstrated the presence of a hypercoagulable phenotype in severe COVID- as well as impaired pulmonary perfusion likely caused by pulmonary angiopathy and thrombosis. | 14 |
| Ackermann M et al | In this study Ackermann et al examined lungs from patients who died from Covid-19 and compared them with lungs obtained from patients who died from A(H1N1) infection as well as uninfected control lungs. This paper demonstrated for the first time Intussusceptive Angiogenesis as a feature of Covid-19 infection. | 16 |
| Rovas A et al | This paper demonstrated alterations of the microcirculation and the endothelial glycocalyx in patients with COVID-19, further implicating systemic vascular alterations in COVID-19 pathogenesis | 18 |
| Burri PH et al | This review elucidates the various aspects of intussusceptive angiogenesis including the processes of pilar morphogenesis, IBR, IMR, IAR. | 26 |
| Medford A et al | This review elucidates the role of VEGF in ALI/ARDS, as well as the putative pneumotrophic roles of VEGF. | 52 |
| Smadja D et al | This paper demonstrated the prognostic role of Angiopoetin-2, as a predictor of admission in ICU and death. This reinforces that endothelial activation is likely a major contributor in COVID-19 pathology. | 58 |
| Pang J et al | This study evaluated the efficacy of Bevacizumab, an anti-angiogenic drug, as add-on therapy for COVID-19. Although the results are promising, further studies are needed to evaluate the role of IA in Bevacizumab's efficacy. | 78 |
Studies suggest that vascular disease plays a major role in Covid-19 course, influencing both susceptibility and outcomes of the infection. Classical cardiovascular risk factors such as hypertension, cardiovascular disease, diabetes, and obesity are the most prevalent comorbid conditions among Covid-19 patients.2,3 These conditions are associated with worse outcomes and are independently associated with Covid-19 related deaths.2,3 Furthermore, they also correlate with age, as ageing is associated with complex vascular changes that result in endothelial dysfunction. Age is the strongest predictor of Covid-19 mortality, implying that endothelial dysfunction might be responsible for part of the excess mortality in the elderly.2,3
SARS-CoV-2 infection results in the disruption of endothelial homeostasis with loss of Renin-angiotensin-aldosterone system (RAAS) balance, anti-thrombotic and immune functions.6 The hyperinflammatory state potentiated by SARS-CoV-2 infection is deleterious to EC. High circulating cytokines levels (the cytokine storm context) act as endothelial activators shifting EC towards a pro-inflammatory, chemotactic phenotype with high permeability and pro-thrombotic features.6,7 A maladaptive innate immune response is also responsible for endothelial damage as DAMPs and PAMPs-mediated TLR activation promotes oxidative stress.8 Neutrophil extracellular traps are also associated with endothelial injury.9
These immune changes along with RAAS dysregulation are associated with hypercoagulability state.10 SARS-CoV-2 infection leads to loss of ACE2 activity in ECs,11 which reduces angiotensin II metabolism and inactivation and consequently lower levels of Angiotensin1-7 (the byproduct of angiotensin II degradation). Higher levels of ATII associated with lower levels of AT1-7 cause vasoconstriction, leucocyte and platelet adhesion, thus promoting thrombogenicity and suppressing fibrinolytic activity.12 Furthermore, ATII regulates NADPH oxidase 2 and higher levels of ATII result in increased oxidative stress,13 further amplifying vascular dysfunction.
This prothrombotic milieu has analytic repercussions. Patients with Covid-19 exhibit increased levels of fibrinogen, D-dimer and fibrin degradation products, von Willebrand factor/factor VIII and lower levels of Plasminogen Activator Inhibitor 1 (PAI-1).10 Data suggests that these mediators correlate with disease severity and thrombotic risk.10,12 This hypercoagulability state is an important part of the pathophysiological basis of cardiovascular complications in Covid-19 patients, such as myocardial injury and dysfunction, deep venous thrombosis, pulmonary thromboembolism and some obstetric complications in pregnant women with SARS-CoV-2 infection such as pre-eclampsia and HELLP syndrome.6
Altogether, these findings provide strong evidence that SARS-CoV-2 infection results in the development of vascular disease.
Pulmonary vascular bed alterations in Covid-19An important feature of COVID-19 associated coagulopathy is microcirculatory endothelial damage in pulmonary vascular beds. Although less well characterized, peripheral pulmonary vascular repercussions are an important pathogenic factor that may result in increasing perfusion-ventilation mismatch, ultimately leading to worsening hypoxemia.14
Covid-19 associated coagulopathy is associated with two main forms of thrombotic repercussions in the pulmonary vascular bed. As Covid-19 is associated with a pro-thrombotic phenotype, the risk of deep vein thrombosis or pulmonary thromboembolism is significantly higher and is a potential cause of acute exacerbation in patients.10 However, the most common form of thrombosis is microangiopathic thrombosis in the pulmonary microcirculation. Studies report microthrombosis features in pulmonary capillaries.15,16 In fact, microthrombi in the pulmonary vascular beds are 9 times more common in SARS-CoV-2 infection than in Influenza infection16 and are associated with worsening hypoxia, shunting and increasing pulmonary vascular resistance. Changes in pulmonary microvascular resistance, more specifically in venules, is responsible for the discrepancy between the relatively preserved ventilatory mechanics and the severity of hypoxemia in Covid-19 patients, which differs from Acute Respiratory Distress Syndrome (ARDS) caused by other agents.17 Microangiopathic thrombosis is also responsible for up to 90% reduction in capillary density in lungs of patients with Covid-19.18
This microangiopathic phenomenon has been demonstrated by histopathological and ultrastructural studies. Typically, pathological evidence shows thrombi in pulmonary arterioles associated with diffuse alveolar damage and hyaline membrane formation.15 The latter are a direct repercussion of increased permeability in the microcirculation and disruption of intercellular junctions of the pulmonary endothelium. Hyaline membranes impair alveolar-capillary barriers and jeopardize gas exchange, contributing to hypoxemia and V/Q mismatch.
Besides the thrombotic manifestations in pulmonary vascular plexus, Covid-19 is associated with features of small vessel vasculitis.16 This is not exclusive to the lung, and Kawasaki-like cases of coronary vasculitis have been described.19 Nevertheless, in the pulmonary vascular beds, SARS-CoV-2 infection promotes angiocentric inflammation. Patients with SARS-CoV-2 infection, especially those with ARDS, exhibit features of vascular acute inflammatory infiltrates with perivascular T-lymphocytes infiltration.16 These inflammatory infiltrates contribute to endothelial barrier disruption that leads to hyaline membrane formation and aggravate respiratory failure. Interestingly, Covid-19 patients display a feature similar to solid organ rejection characterized by a pattern of a T-lymphocyte crown surrounding a highly activated EC. This interaction between immune environment and endothelium has been termed endothelialitis.16,20 These features are highly common in Covid-19 patients and impair the pulmonary vascular bed.
Angiogenesis and Covid-19Ackermann et al analyzed 7 lungs from people who died from Covid-19 and compared them to lungs from patients who died from Influenza and healthy controls.16 They found significant distortion of the lung angioarchitecture with prominent variations in small vessels caliber. Surprisingly, capillaries in Covid-19 subjects lungs exhibit cylindrical microstructures in the capillary lumina, implicating, for the first time, intussusceptive angiogenesis (IA) in the pathogenesis of Covid-19. IA offers the advantage of being faster and more efficient than sprouting angiogenesis (SA) in expanding a vascular plexus,21 promoting arborization through intussusceptive arborization (IAR), optimizing the microangioarchitecture by pruning ineffective or occluded branches (IBR). However, the findings of Ackermann et al must be taken with caution. As Hariri et al point out, sample size is too small to make any extrapolation, as ARDS and Covid-19 are too heterogenous entities.22 But an interesting hypothesis is that these vasculocentric features represent a particular ARDS endotype. Angiogenesis has been implicated in ARDS pathology in subgroups of patients, in the pre-Covid era.23 Nevertheless, this is the first time that IA has been described in the context of ARDS.
The populations compared in the study had major differences, as pointed out by Ackermann and his colleagues.16 In the Covid-19 group, no single patient had been mechanically ventilated, whereas in the Influenza group, a significant proportion of the subjects had been intubated without protective lung measures. It is possible that mechanical ventilation has repercussions in microcirculatory dysfunction and angiogenesis.
The authors also suggest the possibility that differences noted between the two groups were attributable to the differences in the stages, as the Influenza subjects had more advanced and extensive diffuse alveolar damage. Contradicting this hypothesis, Ackermann and his colleagues report increasing levels of IA with increasing time of hospitalization and in Influenza it remained significantly lower and relatively constant.
We believe that IA has been overlooked in pathology reports of patients with ARDS and Covid-19. IP cannot be detected by light microscopy requiring corrosion casting or scanning electron microscopy to be identified, which are rarely used techniques. Furthermore, IA remains largely unknown and poorly studied. IP could be misidentified as artifacts or simply overlooked. Therefore, researchers should be aware of this phenomenon when conducting pathology studies in Covid-19 populations.
Overview of IA mechanisms and roleIA was first noticed in 1986 by Caduff et al while studying angiogenesis in the postnatal rat lung.24 These authors noticed small holes in sheet-like regions of the microvasculature which enlarge to constitute a microvascular network.25 IA is a dynamic process of microvascular growth and development through the formation of IPs that span and divide capillaries lumen forming neovascular networks. Transluminal pillar morphogenesis is the hallmark of IA.26 Pillar development starts with the protusion of diametrically opposed capillary walls into the lumen until direct contact between EC in both sides is established. The result of this protrusion is the formation of a transluminal interendothelial cellular bridge. Further reorganization of the interendothelial junction results in a perforated core within the endothelial bilayer which is occupied in a centripetal fashion by cytoplasmatic processes of fibroblasts and pericytes. Pericytes and fibroblasts then secrete collagen fibrils further dividing the initial single lumen into two.27 Alternative morphogenic mechanisms of pillar formation has been described27 and include pillar formation by kissing or peg-like contacts, meso-like intravascular folds, merging of adjacent capillaries or splitting of intercapillary meshes.
Mechanistically, IA can be divided into three different processes: intussusceptive microvascular growth (IMG), IAR and intussusceptive branching remodeling (IBR), which occur in tandem during embryogenesis. In post-natal organs however, they overlap in time and constitute a single process.26,27
IA starts with IMG, which initiates pillar morphogenesis and expansion, allowing a quick capillary plexus development. This constitutes a primordial vascular bed characterized by an increased surface area. IMG is a ubiquitous mechanism28,29 allowing rapid expansion of capillary plexus without jeopardizing vascular and hemodynamic efficiency.28 This quick vascular expansion is particularly useful in tissues with high metabolic demands, ischemia or hypoxia allowing nutrients and oxygen to be readily delivered and metabolites to be swiftly removed.30 IAR then allows the establishment of the proper angioarchitecture by remodeling the capillary bed in an organized, branched.28,29 Ultimately, IBR increases the efficiency of nutrient and gas exchange by remodeling branches in poorly oxygenated areas and pruning branches that are superfluous or inefficient.28,29
Evidence of IA was found during the formation and remodeling of vascular beds in many organs including: the mammary gland, the bone, the glomeruli, the skeletal muscle, the ovaries, and others.31-37 This ubiquity of the process of IA in the human body was not exclusive of physiological mechanisms and soon, evidence of IA in pathological scenarios arose, including both non-neoplastic and neoplastic diseases.38-44 This data proves that IA is a relevant form of angiogenesis, playing a pivotal role in a wide variety of settings. However, its stimuli, molecular mechanisms, whether it occurs in synergy with other vascularization processes, and whether it plays an advantageous role in pathological conditions remain poorly understood.
Possible hypothesis on the occurrence of IA in Covid-19 patientsEvidence suggests that IA occurrence in Covid-19 results from the dynamic interaction between different factors:
- 1.
Hypoxia, VEGF and angiopoietins:
In ARDS, inflammatory markers (e.g. IL-1) and neutrophilic mediators (e.g. ROS, elastasis, LPS) cause extensive damage to the alveoli capillary plexus and endothelium. Such damage leads to transudation of fluid to the interstitium and air spaces during exudative phase and, later, exudation of neutrophils and protein-rich fluid, culminating in the formation of hyaline membranes that impair gas exchange and ultimately cause hypoxemic respiratory failure with regional alveolar hypoxia.45
Hypoxia is a known angiogenesis promoter.46 Local hypoxia associated with ongoing inflammatory processes results in HIF-1α stabilization. HIF-1α regulates the expression of a wide range of genes involved in vasodilation, extracellular matrix remodeling, angiogenic pathways such as the VEGF and Angiopoietin/Tie2.47
Viral infections (e.g HCV, EBV, HPV) per se induce HIF-1α activation.48 This phenomenon seems to be dual as HIF-1α also contributes to the pathogenesis of the infection itself. Interestingly, HIF-1α overexpression reduces ACE2 membrane expression.49 However, this effect is damped if AT II is inhibited, suggesting a close relationship between HIF1α and AT II.48 This might explain why populations living under chronic hypoxic conditions (e.g. Himalaya and the Andes) display less severe disease features.50 Thus, it is likely that HIF-1α is induced in Covid-19 associated ARDS. Nevertheless, its role in IA is unknown.
VEGF and its receptors are widely expressed in the lung (mainly in the alveolar epithelium) even in a non-pathological state, suggesting a potential physiological role of VEGF in the lung.50 In fact, VEGF acts as a stimulant and mitogen of the alveolar epithelium52 and primary human type 2 alveolar epithelial cells express VEGFR252 which implies a possible autocrine role of VEGF in the air space beside its paracrine actions in vascular bed.52 VEGF also plays an important role in pulmonary organogenesis as VEGF antagonism during the embryonic period arrests alveolarization besides leading to impaired lung vasculogenesis.52 Conversely, transgenic HIF-2α−/− mice (leading to VEGF down-regulation) have diminished surfactant production and die from neonatal respiratory distress syndrome (RDS). Interestingly, in utero administration of VEGF165 to these mice is protective against RDS.52 Post-natal, VEGF-R2 blockage results in emphysema and alveolar apoptosis without inflammatory features,52 implying that VEGF pneumotrophic role is not only important during embryogenesis but also in the post-natal life.
VEGF has been implicated in the pathogenesis of ARDS before the pandemic. In fact, VEGF seems to be a key regulator of the interstitial edema that happens in ARDS. This edema occurs despite normal pulmonary capillary wedge pressure and normal oncotic pressure, which implicates endothelial permeability as the main culprit. VEGF is one of the prominent mediators of vascular permeability. VEGF/VEGFR2 interaction results in an intracellular cascade that among other factors, results in the downstream activation of nitric oxide synthase (NOS) and Rho-Rac pathway with subsequent involvement of junctional signaling proteins leading to increased vascular permeability.53 Gene therapy delivering VEGF165 resulted in non-cardiogenic lung edema and an increased capillary permeability, a phenotype very similar to that of ARDS.52 Pro-inflammatory stimuli such as LPS and neutrophil-derived enzymes stimulate VEGF secretion by alveolar cells and acute lung injury (ALI)/ARDS models demonstrated increased VEGF levels in the lung, early after insult, simultaneously to increased protein levels and neutrophils in bronchoalveolar lavage.54 This suggests a pivotal role of VEGF in exudate and hyaline membranes formation phases of ARDS. Yet, typically, there is a concentration gradient through the alveolar-capillary membrane with VEGF concentration in the air space being 500 times higher than in the capillary. In ARDS, however, there is a shift of this ratio and even though plasma VEGF levels increase, there is a marked reduction of the levels in the air space.51 It is possible that this reduction of VEGF results in a loss of its pneumotrophic protection and aggravation of alveolar damage.
Besides its role in vascular permeability, VEGF signaling probably plays an important role in Covid-19-associated IA. VEGF family members and their pathways are the best studied angiogenic stimulating factors promoting mitogenesis, differentiation and migration of ECs. VEGF plays a critical role in both SA and IA29 and its actions are therapeutically modulated in settings where angiogenesis is contributing to the pathogenesis of the underlying condition (e.g. retina neovascularization and tumor neoangiogenesis).55 Its role in IA was demonstrated in models of the chorioallantoic membrane (CAM)26 as well as in human skeletal muscle.56,57 VEGF signaling in SA and IA depend on a multitude of factors such as experimental model, dose, location, process.56 While high levels of VEGF promote sprouting, lower levels of VEGF may be crucial in promoting intussusception. Such dose-dependent effect explains the transient increase in intussusception in tumor neovascular beds after treatment with anti-VEGF therapies. However, in skeletal muscle, high levels of VEGF are associated with IA mainly because there is a disruption of the concentration gradient, therefore promoting a switch from SA to IA.56,57 Reduced levels of VEGF are also associated with vascular pruning and may play a role in IBR.28 In Covid-19 patients, high VEGF plasma levels have been reported from early stages.57 This is consistent to previous findings in ARDS patients. It is, therefore, likely that disruption of the alveolar-plasma VEGF gradient in Covid-19 patients aggravates diffuse alveolar damage already in the early stages of disease. Although VEGF concentration gradient disruption may promote shifting from SA to IA, as IA seems to be a late feature, associated with prolonged hospitalization, it is unlikely that VEGF per se can cause angiogenesis by splitting. Other factors may as well contribute to IA in Covid-19 patients.
VEGF overexpression associated with Angiopoietins-1/2 overexpression results in a higher number of small holes in primordial capillary plexuses, a sign of increased IA.29 Angiopoietins are important signaling molecules for EC-pericyte crosstalk. Ang-2 secretion by EC is stimulated by hypoxia (the hallmark of ARDS), TNF-α (typically elevated in Covid-19), turbulent flow and thrombin. Therefore, Ang2 is a marker of endothelial activation and associates with endothelial dysfunction.58 In Covid-19 patients, Ang2 levels rise along with VEGF levels, probably reflecting extensive endothelial dysfunction and activation.58 High levels of VEGF and of Ang-2 in the latter stages of Covid-19 could be the molecular drive to promote IA. While VEGF may act as a promoter of IA, Angiopoietins may play a crucial role in the subsequent phases.29 Ang-2 acts as an Ang-1 antagonist and promotes vascular regression, disrupts vessel integrity and promotes EC death. Ang-2 also acts with PDGF to promote pericyte division, migration and recruitment, which is important in pillar morphogenesis.29 This is corroborated by the fact that Tie2-knockout mice have impaired pillar morphogenesis.29 Angiopoietin expression is modulated by shear stress. Accordingly, laminar shear stress down-regulates Ang-2 expression while turbulent shear stress promotes the opposite.29
- 2.
Microangiopathic thrombosis, hemodynamic and platelet derived factors:
Covid-19 is associated with extensive microangiopathic thrombosis. Obstruction of pulmonary microcirculation results in significant changes in hemodynamic conditions. The MYSTIC study18 reported significant reductions of VRBC associated to capillary density loss in Covid-19 patients. This loss is almost exclusive to small capillaries between 4–6 µm diameter and results in a lower area of gas exchange, contributing to worsening hypoxia. D-dimers correlate strongly with vascular density, indicating a causality between microthrombi and vascular regression. This could be explained by a mechanism of intussusceptive branching remodeling and pruning. Microthrombi result in vasooclusion and acute reductions in blood flow, shear stress together with high transmural pressures caused by the thrombi promote IBR, which optimize the disrupted angioarchitecture, reconducting blood flow to spared areas.
Local changes in hemodynamic forces remarkably impact in IA mechanisms. It has been demonstrated that IMG and IAR occur predominantly in regions with high blood flow.59-60 Blood flow redistribution to patent branches results in areas of high blood flow with high distending forces promoting IP morphogenesis and high shear stress promoting arborization of the newly formed branches by IAR.
Apart from hemodynamic factors, platelet derived factors may also play a role in IA. PDGF-BB markedly accelerates the splitting process.56 PDGF-BB modulates VEGFR-2, promotes pericyte survival and controls vascular circular expansion.57 This mechanism protects against aberrant angiogenesis promoted by exacerbated VEGF.55,56
- 3.
Inflammation, endothelialitis and cytokine storm:
The immune system also plays an important role in IA. Mononuclear cells stabilize IP by protruding uropod-like projections and producing collagen.61 The role of mononuclear cells such as lymphocytes or monocytes was first described in a Notch knockout model.61 Notch inhibition has been associated with mononuclear cell recruitment and migration as well as with increased pillar morphogenesis. While the precise mechanism is unclear, this process is known to depend on chemokines such as CXCL12 or SDF-1.61,62 SDF-1 is responsible for the Notch-dependent mobilization of mononuclear cells acting in MMP-9.63 Furthermore SDF-1 also acts in CXCR4 receptor in mononuclear cells to promote the formation of the uropod-like that stabilize the IP's.63
VEGF and bFGF promote IA by acting on the SDF-1/CXCR4 pathway.62 In fact, a study using a mouse model of diabetic retinopathy reported increased retinal neovascularization after vitreal injection of SDF-1.64 Hypoxia, a known angiogenic trigger, promotes SDF-1 up-regulation and SDF-1/CXCR4 pathway seems to play an important role in re-establishing blood flow to ischemic zones through IA.61-63
SDF-1/CXCR4 are associated with T-lymphocyte infiltrates in Covid-19.65 It is therefore likely that a positive feedback between shear stress alterations induced by inflammation (particularly T-cell), SDF-1, hypoxia and eNOS promote angiogenesis. This represents a vascular adaptation to maintain high blood flow prominent inflammatory areas. A dynamic interaction between T-cell infiltrates and EC, a phenomenon of endotheliatis has been described in Covid-19. This further contributes to prolonged local inflammation, enhancing thus angiogenesis. In endothelialitis, T-cell infiltrates activate ECs, which overexpress TLRs and MyD88. These molecules recognize SARS-CoV-2 and promote cytokine, chemokine and adhesion molecule expression as well as angiogenic factors namely VEGF, NOS, and CD14 monocytes recruitment.65
One of the most prominent features of COVID-19 is an inappropriate and excessive inflammatory response with concomitant release of large amounts of pro-inflammatory (e.g. IL-6 and TNF-α), a process termed “cytokine storm” (CS).66 CS relates to the severity of Covid-19 and is directly correlated with lung injury, multi-organ dysfunction and adverse outcomes in Covid-19 patients.66-67
IL-6 is the most frequently reported cytokine elevated in Covid-19 and its levels are independently associated with higher mortality.67 IL-6 has been implicated in pathogenic angiogenesis in different scenarios (rheumatoid arthritis, stroke and cancers). Studies in tumors have shown that IL-6 levels are directly correlated with VEGF levels and vessel density.68 The published literature suggests that IL-6 can drive aberrant angiogenesis.69 IL-6 promotes the formation of defective vascular beds with abnormal pericyte coverage. Loss of pericytes has been described in Covid-19,70 however it was postulated that this was a direct effect of viral infection as pericytes express ACE2. We suggest that IL-6 might be a contributor to this shift. Loss of pericytes has been previously implicated as an angiogenic driver, promoting both SA and IA.71 Thus, the aberrant effects of IL-6 on pericytes could be related to IA in Covid-19.68 Furthermore, studies showed that IL-6-trans-signaling inhibits proliferation and tube formation of ECs that could also explain the shift from SA towards IA in Covid-19.71
Taking into account the complex etiopathogenesis of Covid-19, we anticipate that the development of IA is attributed to the intricate involvement of hypoxia, proangiogenic and platelet-derived molecules, microangiopathic thrombosis, hemodynamic forces and inflammatory factors in these patients.
Prognostic and therapeutic implications of IA in Covid-19Ackermann et al reported increasing density of angiogenic features with increasing duration of hospitalization, suggesting IA as a potential prognostic tool in Covid-19 patients. Angiogenic mediators have an established prognostic role in ARDS. Positive correlations between VEGF, soluble VEGFR2 (sVEGFR2), Ang2 and Ang2/Ang1 ratio and ARDS development in critical illness have been described.54 Moreover, Ang2 and sVEGFR2 are also associated with adverse outcomes and higher risk of mortality in ARDS patients.54,72
Ang2 is a marker of endothelial activation.58 Ang2 levels have been consistently reported elevated in critical Covid-19 patients. High levels of Ang2 are also associated with poor lung compliance, higher CRP and D-dimers,58,73,74 thus reflecting CAC in Covid-19 patients. Ang2 is associated with worse outcomes in Covid-19, predicting ICU admission, mechanical ventilation, and death.58,74 Therefore, Ang2 is a strong prognostic biomarker in critical Covid-19 patients. However, whether that reflects only endotheliopathy and EC activation or also IA is unknown. It is possible that EC activation triggers Ang2 secretion which in turn promotes angiogenesis, therefore linking endothelial dysfunction and IA. It is also unknown if Ang2 levels correlate with IA in critical Covid-19 patients, although that hypothesis seems likely.
Moreover, data also suggests that VEGF-A and Flt-1 levels are elevated in both non- and critical patients.75 VEGF-D levels are lower in patients in mechanical ventilation than those who are not submitted to this procedure.76 However, plasma VEGF levels must be analyzed with caution as plasma VEGF changes may not reflect local VEGF changes and VEGF may be sequestered by tissues as most VEGF isoforms are heparin binding.
Other endothelial dysfunction markers have been described in critical ICU patients such as follistatin, PAI-1, E-selectin and vWF.73-75 These markers are also higher in patients that died than survivors, suggesting a potential prognostic role.
Biomarkers of angiogenesis are also associated with other factors such as vascular permeability and endothelial dysfunction. Further studies should focus on confirming whether IA is an independent prognostic factor in Covid-19. Direct quantification of angiogenic features would be a better way of establishing this process, than using indirect biomarkers such as Ang or VEGF because the latter are susceptible to many factors implicated in Covid-19’s pathogenesis.
Therapeutic modulation of IA is a potential adjuvant therapy in Covid-19. Among the therapeutic strategies implemented in COVID-19, a few are known to inhibit angiogenesis. Glucocorticoids alone or in combination with other pharmacological agents have been largely used to treat SARS-CoV-2. Corticosteroids are well known to exert anti-angiogenic effects in many disorders including cancer and vasoproliferative ocular disorders.77 Currently, the anti-angiogenic drug, bevacizumab is being studied in Covid-19 (NCT04344782, NCT04305106 and NCT04275414). Bevacizumab is a monoclonal antibody that targets VEGF-A and inhibits its actions, used in oncotherapy of different neoplasms with efficacy and safety.78 A recent single-arm trial (NCT04275414) suggested beneficial results of using Bevacizumab as an add-on therapy.79 They report improvements in oxygenation (reflected by PaO2/FiO2 ratio, oxygen support), radiologic improvement, fever and lymphocyte count. Although anti-angiogenic, IA degree was not taken into consideration in this study. The authors suggest that the beneficial effects of bevacizumab in oxygenation are related to modulation of vascular leakage and diminishing pulmonary edema (a rationale similar to the effect of bevacizumab in capillary-leakiness in age-related macular degeneration). Interestingly, they report positive outcomes of fever resolution and lymphocyte count. VEGF has a speculative role in immunopathology, promoting inflammatory cell mobilization to pathological tissues. Therefore, bevacizumab's anti-pyretic effect could be related to antagonism of inflammation caused by SARS-CoV-2 infection, as bevacizumab also resulted in lower CRP levels in Covid-19 patients. Another striking finding is that bevacizumab improves lymphopenia, associated with worse outcomes in Covid-19. Although the exact mechanism is unknown, it is hypothesized that bevacizumab might affect lymphocyte extravasation and redistribution. Further randomized control trials are needed to establish the use of bevacizumab in Covid-19 and to assert its effects on intussusception. Nonetheless, these findings are promising, and pave the way for the use of anti-angiogenic therapeutic agents against Covid-19.
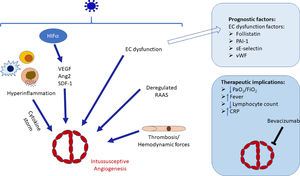
ConclusionThe pathogenesis of SARS-CoV-2 infection is complex and different pathogenic factors contribute to the severity of the infection. Among those, vascular repercussions of the infection are a major threat, severely worsening prognosis and significantly increasing mortality. Notwithstanding the fact that a lot of effort was put into studying these vascular repercussions (including the endothelial dysfunction), intussusceptive angiogenesis, a prominent feature of Covid-19, remains poorly understood. There are significant gaps in knowledge regarding splitting angiogenesis including molecular control and regulation, microenvironmental factors and therapeutic and prognosis implications. The current paper reviewed the main characteristics and mechanisms of IA and formulated a theoretical model that explains the occurrence of splitting angiogenesis in Covid-19 (Fig. 1). We conclude that although there is much to learn regarding this subject, IA is likely to be the product of hypoxia, classical angiogenic molecular factors (e.g. VEGF, Ang-2), hyperinflammation and cytokine storm, thrombosis and associated hemodynamic changes and RAAS dysregulation. To the best of our knowledge this is the first comprehensive review regarding this intriguing feature of Covid-19.
Schematic diagram of the stimuli interactions that drive intussusceptive angiogenesis occurrence in COVID-19. In a synergic way, hypoxia-induced angiogenic factors, including VEGF, SDF-2 and Ang-2, hyperinflammation and cytokine storm, thrombosis and associated hemodynamic changes and RAAS dysregulation will trigger intussusceptive growth in COVID-19 patients. Factors implicated in endothelial dysfunctions may be used as prognostic factors. Splitting angiogenesis may therefore be a therapeutic target in COVID-19. EC, Endothelial cell; HIF1α, Hypoxic-inducible factor 1α; VEGF. Vascular endothelial growth factor; Ang2, Angiopoietin-2, SDF-1, Stromal-derived factor-1, RAAS, Renin-angiotensin-aldosterone system; PAI-1, Plasminogen activating inhibitor-1; E-selectin, Endothelial-leukocyte adhesion molecule; vWF, von Willebrand factor; CRP, C reactive protein.
The authors declare that there are no conflicts of interest.
The authors acknowledge Joao Incio for the English revision. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.