Pulmonary alveolar proteinosis (PAP) is a rare syndrome characterized by the alveolar accumulation of surfactant due to a deficient macrophage activity, resulting in impaired gas exchange.1
PAP can be classified into different types. Autoimmune PAP is the most common (90–95%), and results from autoantibodies against granulocyte-macrophage colony-stimulating factor (GM-CSF).1,2 Secondary PAP is related with an underlying condition.1 Additional forms of PAP, such as Congenital, are associated with genetic abnormalities.1,3
The diagnosis of auto-immune PAP is based on three findings: crazy paving pattern on high resolution chest tomography (HRCT) as a consequence of intra and interlobular septal thickening superimposed on patchy ground-glass opacities; positive GM-CSF autoantibody; and bronchoalveolar lavage (BAL) with milky appearance and finding of lipid-rich proteinaceous material, positive to periodic acid-Schiff stain. Lung biopsy may be required if the previous findings are indefinite.1
Therapeutic decisions are made in accordance with PAP classification and disease severity.1 Whole-lung lavage (WLL) has been the cornerstone therapy,1 but treatment with inhaled GM-CSF has shown improvement in measures of pulmonary gas transfer, functional health status and pathologic features, when compared to placebo.2 Corticosteroid therapy suggested more damage than gain.1
Hypersensitivity pneumonitis (HP) is an immunologically mediated interstitial lung disease, caused by repeated exposure to inducing environmental agents in a genetically predisposed individual. Its diagnosis relied on a multidisciplinary discussion focused on typical chest CT scan, histological findings and high lymphocytosis in BAL.4 Although HP may be triggered by a comprehensive number of antigens, the antigen responsible is often not identified, and chronic forms of HP can even be indistinguishable from idiopathic pulmonary fibrosis.5,6
The authors present the case of a 43-year-old Caucasian male, former smoker (10 pack-year), volunteer firefighter, and with medical history of peptic ulcer, that presented in 2011 a 12-month history of progressive exertional dyspnoea and cough. Symptoms initiated after exposure to smoke. He denied fever, weight loss, arthralgia, haemoptysis or chest pain. No history of infections, malignancy or previous respiratory lung disease. Physical examination was unremarkable.
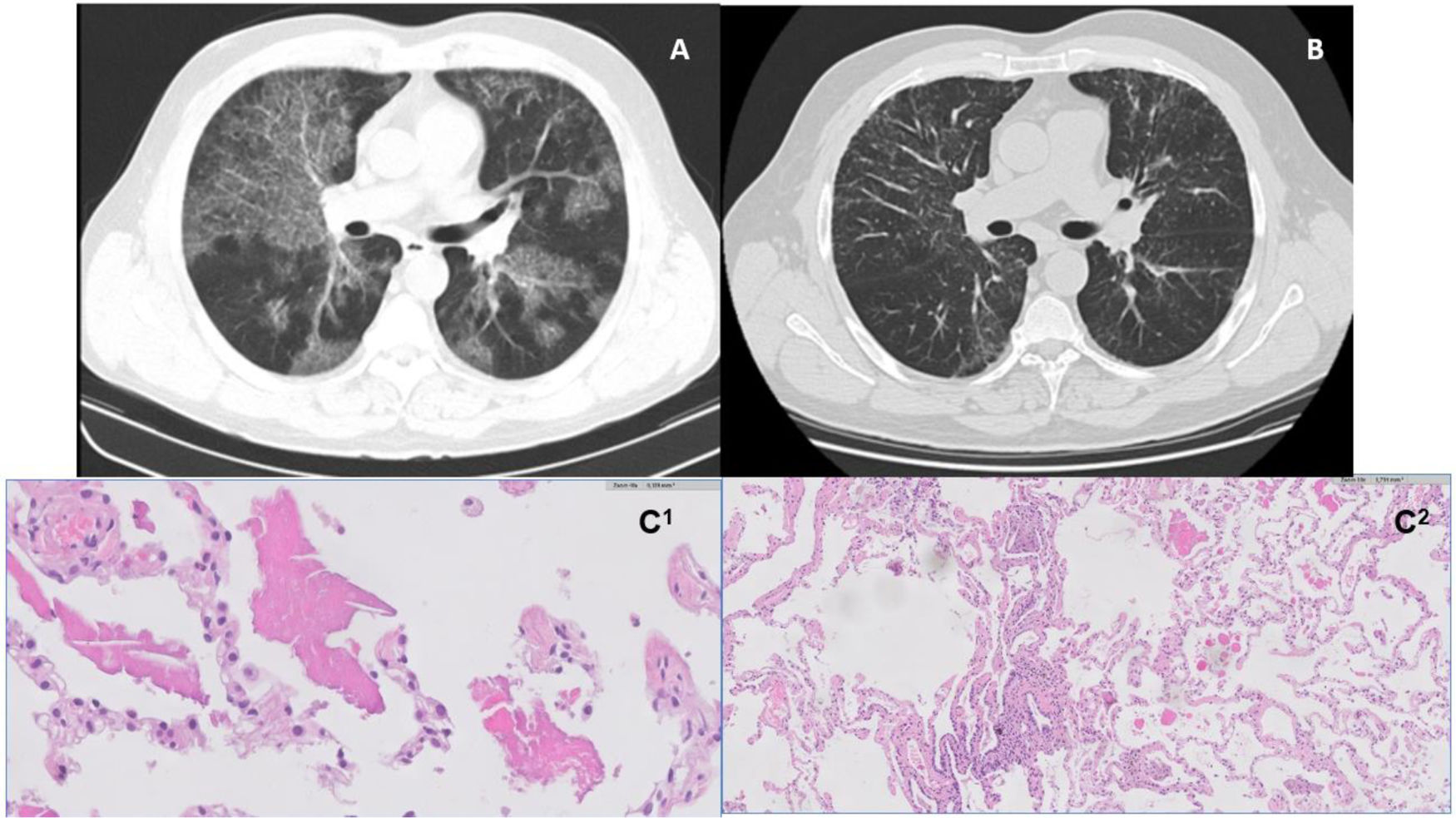
He was diagnosed as having PAP based on chest HRCT findings (Fig. 1A), BAL (significant amount of periodic Acid-Schiff material, namely with various cell bodies) and positive anti-GM-CSF antibodies. Lung function tests showed a mild defect in carbon monoxide transfer factor (TLCO).
A and B – High resolution computed tomography images (axial lung window). On the left, image from 2011, illustrating bilateral ground glass alveolar infiltrates and interlobular septal thickening consistent with a ‘crazy paving’ pattern (A); On the right, image from 2018, with reticular opacity in a manly peripheral distribution, and traction bronchiectasis in the bases (B). C1 and C2 – histology images. On the left, HE 10X – pulmonary alveolar proteinosis (PAP) shows alveolar filling by a proteinaceous material (C1); On the right, HE 10X – cellular interstitial pneumonia airway-centered and peribronchiolar metaplasia; multinucleated giant cell (C2).
In 2013 he presented symptomatic, functional (PaO2 74mmHg; shunt fraction 20.8%) and radiological deterioration; therefore, therapeutic WLL was carried out, resulting in symptom and gas exchange improvement.
Five years later, despite clinical and functional stability, chest HRCT (Fig. 1B) revealed a shift in its pattern, characterized by peripheral and peribronchovascular reticulation, and traction bronchiectasis. New flexible bronchoscopy with BAL analysis was performed, revealing intense lymphocytosis (56,4%), with cd4/cd8 ratio of 1,7. BAL was translucent and negative for periodic Acid-Schiff (PAS) granules.
New occupational and environmental exposure to organic antigens were pursued; serum specific IgG antibodies (avian and fungus) as well as autoimmune screening were repeated; all were found to be negative.
After multidisciplinary discussion, a transbronchial lung cryobiopsy was performed; histology revealed features of alveolar proteinosis consisting of intra-alveolar eosinophilic material and coexisting features consistent with hypersensitivity pneumonitis (Fig. 1 – C1 and C2).
PAP and HP are known interstitial lung diseases, however completely different entities with distinct clinical features. An overlap phenotype between HP and PAP has already been described in a published article reviewing five cases, where patients show concurrent radiological and histopathological features of both diseases.7 Nonetheless, to the best of our knowledge, this is the first case presenting this sequence of events – PAP initially, with HP appearing later on.
We hypothesized that the patient always presented positive GM-CSF antibodies and the exposure to smoke or silica extinguisher powder acted as a trigger to develop PAP; it is known that inhalation of toxic dusts and fumes is a major risk factor for developing secondary PAP.8 Many patients with autoimmune PAP have a history of exposure (23%), and positive autoimmunity may be present in some secondary PAP cases.9 At least two recognized cases reporting PAP secondary to extinguisher particle’s exposure, are described in the literature.10,11
Curiously, our patient developed HP later on, once PAP was under control. We postulate that the development of HP might be explained by the capability of PAP itself to predispose to a secondary pathology, causing a lung hypersensitivity reaction.7 It is possible that alveolar epithelial damage following PAP might induce susceptibility to environmental triggers, especially if there is an underlying immune dysregulation. Our patient had positive GM-CSF antibodies and some studies demonstrate that GM-CSF antibodies have an important role in reducing lung injury after insult.12 High values of GM-CSF antibodies were found in patients with HP and sarcoidosis who also presented various features allusive of autoimmune PAP. This finding suggests that measurement of GM-CSF antibodies could help in identifying concomitant early-onset autoimmune PAP.13
Another hypothesis is that leakage of PAS positive material from damaged alveoli/bronchus led to a pulmonary response similar to HP.
In conclusion, PAP is a rare entity, with still much to clarify regarding its evolution. We reported the first case of HP after PAP. It is unclear if this association occurred by chance or if PAP predisposed the HP. Longitudinal records and registries may be a helpful tool to better understand the natural history of PAP.
Ethical disclosureThis study was conducted in accordance with Helsinki Declaration, revised in 2013, and approved by the ethical committee of Centro Hospitalar de Vila Nova de Gaia/Espinho.
Authorisation for publishing clinical data was obtained from the patient. Nevertheless, personal data was anonymized.
Conflicts of interestThe authors have no conflicts of interest to declare.