Immunotherapy is an effective treatment option for patients with non-small-cell lung cancer (NSCLC) with advanced disease. Several immune checkpoint inhibitors (ICI) have significantly improved survival of patients with NSCLC.1 The safety profile of immunotherapy differs from the known safety profile of chemotherapy and includes immune-related adverse events (irAEs) such as fatigue, rash, pruritus, diarrhea and arthralgia, occurring in >20% of patients.2 A recent meta-analysis reported all-grade immune-related lung toxicity (pneumonitis) in 4.1% of patients with NSCLC treated with ICIs, which was reported as grade ≥3 in 1.8% of patients.3 In daily practice, it is important to know whether it is possible to rechallenge a patient with NSCLC with the same PD-1 inhibitor after resolution of an irAE to be able to continue providing clinical benefit.
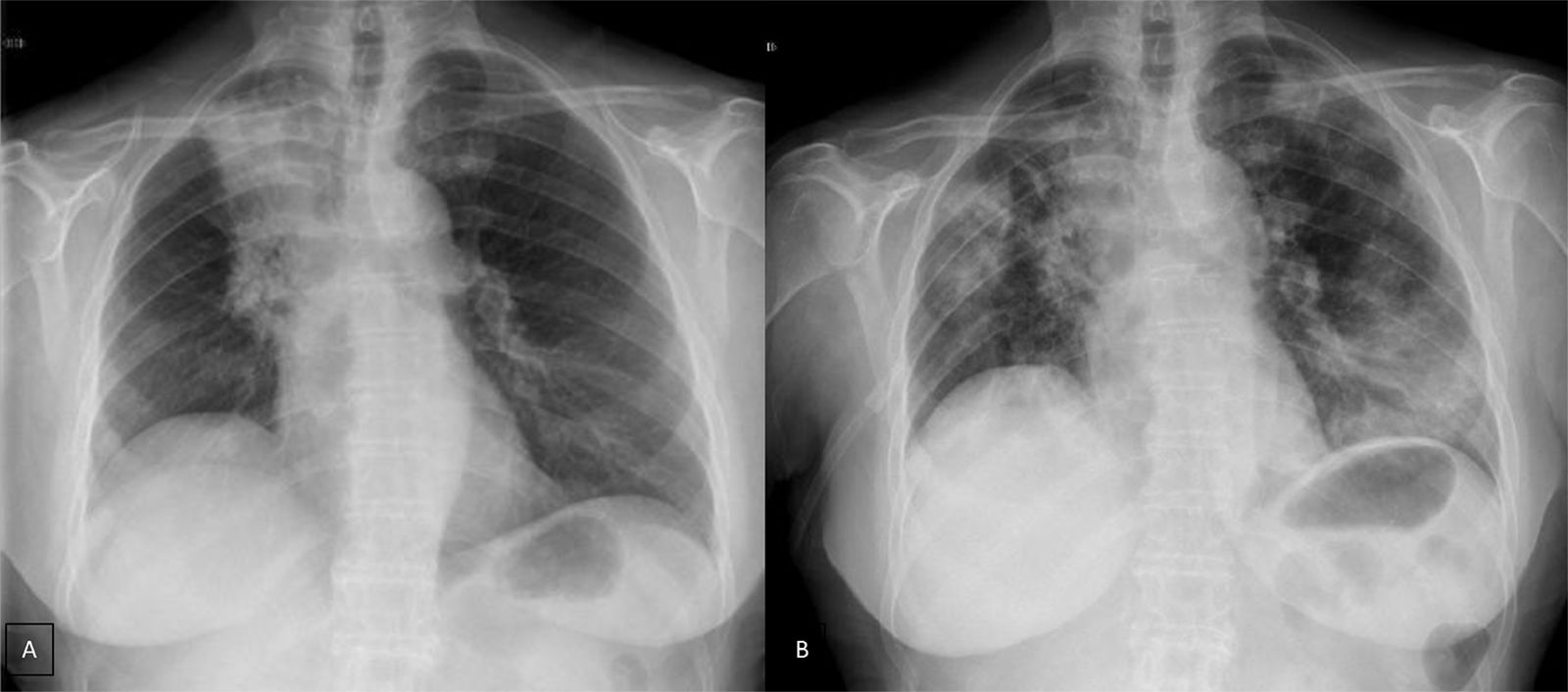
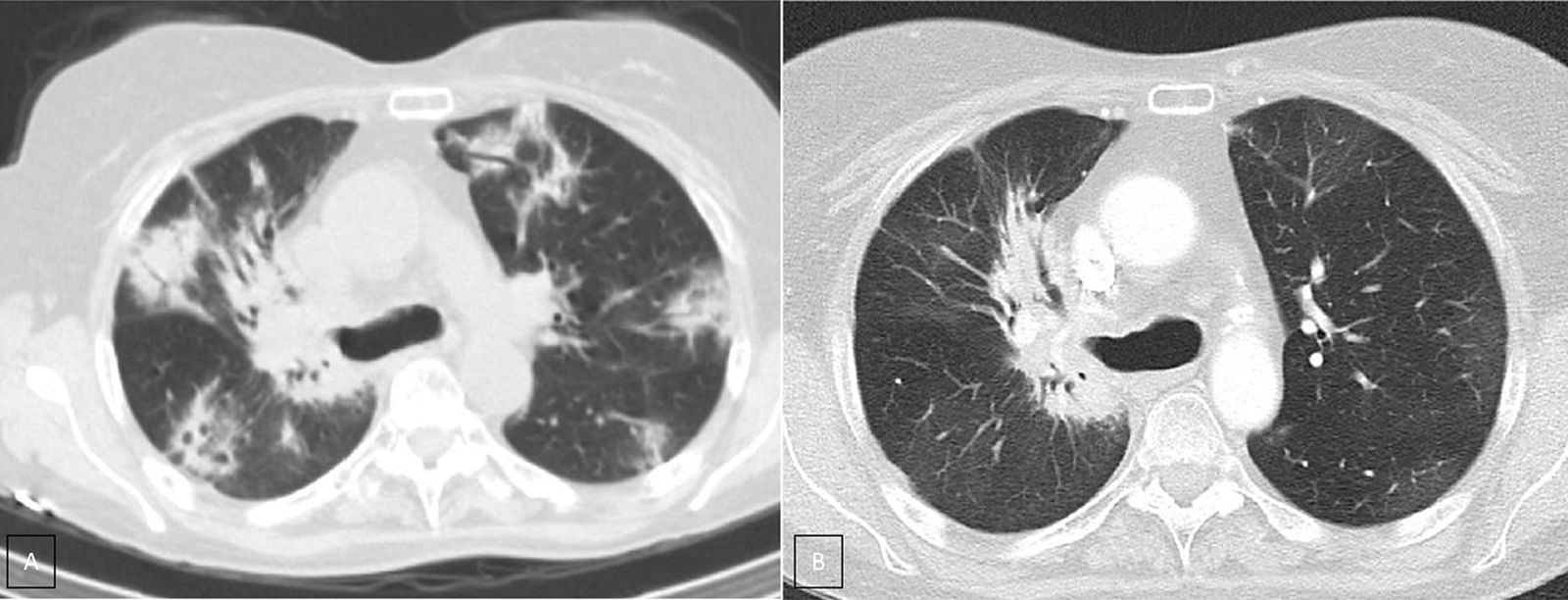
Case presentationWe report the case of a 63-year-old female patient diagnosed in December 2014 with NSCLC (cT2N2M0), localized at the right upper lobe. She received neoadjuvant chemoradiotherapy with intravenous cisplatin 75mg/m2 plus vinorelbine 25mg/m2 on days 1 and 8 in 3-week cycles and 60Gy (in 2Gy per fraction) from January 2015 until April 2015. After completing 2 cycles she was not a candidate for surgery due to the persistence of N2 (evaluated by Endobronchial Ultrasonography [EBUS]), so she continued with 2 more cycles of chemoradiotherapy until May 2015. In January 2016, the patient showed disease progression in the lung (Fig. 1A), so she started a second-line of treatment with nivolumab (3mg/kg intravenously every 14 days) attaining a partial response in March 2016 after 4 treatment cycles. In April 2016, the patient was admitted with grade 3 fever and increased dyspnea that occurred even on minimal exertion. These symptoms had been ongoing for 2 weeks before being admitted to the hospital. An X-ray was performed, showing bilateral dispersed alveolar opacities (Fig. 1B). The main differential diagnoses were infection, immune-related toxicity, radiation-induced pneumonitis, and disease progression. Thoracic computed tomography (CT) (Fig. 2A), bronchoscopy with bronchoalveolar lavage (BAL) and bronchoalveolar aspirate (BAS) were performed to ensure the correct diagnosis. Negative bacterial cultures ruled out the possibility of infection and cytology did not show malignant cells. Although late radiation pneumonitis was difficult to exclude, the time elapsed between the end of the radiotherapy treatment and the beginning of the symptoms suggested an immunerelated pneumonitis. Moreover, irradiated lungs are more susceptible to develop pneumonitis when treated with ICI. The patient was diagnosed with a grade 3 immune-related pneumonitis and the PD-1 inhibitor had to be permanently stopped, despite having attained a partial response. Pneumonitis was treated with high doses of corticosteroids (methylprednisolone 1mg/kg/day) followed by tapering. Patient symptoms improved after 1 week of corticosteroid treatment, with complete clinical and radiological recovery after 11 weeks of treatment (Fig. 2B). On May 2017, during a follow-up visit, a thoracic CT scan showed disease progression. Considering the patient's previous response to immunotherapy, including 14 months of stable disease after stopping treatment, nivolumab rechallenge was proposed as a treatment option despite the toxicity reported. To avoid new irAEs, nivolumab (3mg/kg intravenously every 14 days) was reinitiated along with low dose corticosteroids (methylprednisolone 8mg/day). After four cycles, the patient achieved a partial response in the lung tumor with no further lung toxicity.
A. CT scan (lung window) shows multiple peripheral poorly defined areas of focal consolidation, very suggestive or organizing penumonia. B. CT scan (lung window), three month later shows resolution of peripheral areas of focal consolidation, but persistence of parahilar consolidation because of radiation fibrosis. Note the bronchiectasis and volume loss and the sharp demarcation between normal lung tissue and areas of fibrosis.
Immunotherapy-related lung toxicity is rare but can be life-threatening.2 The clinical presentation of pneumonitis usually consists of non-specific symptoms. However, it is essential to consider pneumonitis among the differential diagnoses in patients receiving treatment with PD-1 or PD-L1 inhibitors, before the respiratory function worsens.3 To correctly diagnose pneumonitis, a bronchoscopy must be performed to rule out other etiologies, such as infection. The main treatment for the irAE of pneumonia is the administration of high doses of corticosteroids (1–1.5mg/kg) with subsequent tapering when symptoms and radiological imaging show improvement.4 Moreover, according to the evidence described in the literature,4,5 permanent cessation of immunotherapy is the standard procedure in a patient experiencing a grade 3–4 irAE. However, clinicians should always consider the potential loss of clinical benefit for patients in these situations. Although the experience described in our patient reflects a single case, other case series have shown that rechallenging with a PD-1 inhibitor could be an option for patients with NSCLC, even after discontinuation due to toxicity.6 Currently, a few trials, such as the REPLAY study (NCT03526887) carried out by the Spanish Lung Cancer Group, are evaluating pembrolizumab in NSCLC that had failed after obtaining benefit from a checkpoint inhibitor. Furthermore, other studies are assessing the risk of recall toxicities when restarting immunotherapy. A study of patients who have been diagnosed with immune-related pneumonitis showed that 25% of these patients experienced a recurrence when rechallenged with PD-1/PD-L1 inhibitor.7 In summary, ICI rechallenge in patients with NSCLC who experienced a grade 3–4 irAE could be an option, although more evidence is needed.