The multiple-breath inert gas washout (MBW) test involves recording an inert tracer gas being cleared from the lungs during normal tidal breathing. In nitrogen (N2) MBW resident nitrogen is washed out by inhaling 100% oxygen. MBW allows us to calculate the lung clearance index (LCI), defined as the number of lung turnovers required to washout an inert gas to 1/40th of its initial concentration.1–3 It offers complementary information to standard lung function tests, such as spirometry1
The procedure is strongly dependent on skilled operators and a relaxed testing environment is key to obtaining good quality measurements.4 Operator training and certification in performance of the MBW measurement is imperative to achieving high-quality data, and standardization of LCI is part of an ongoing collaborative, multicentre process.2
Aim: to assess the feasibility of N2MBW in a paediatric lung function laboratory in children with different clinical conditions [Cystic Fibrosis (CF), primary ciliary dyskinesia (PCD) and healthy controls].
School-aged children with a confirmed diagnosis of CF or PCD were recruited from a Paediatric Pulmonology clinic in a tertiary-care hospital, and healthy controls from the community, throughout December/2018-November/2019. Patients were clinically stable at inclusion and the study procedures were performed during the patients’ routine clinic visits. Height and weight were measured prior to the lung function assessments, and BMI z‐scores standardized using WHO reference values.
Exhalyzer® D and the associated software Spiroware® version 3.1 (EcoMedics AG, Duernten, Switzerland) were utilized for the N2MBW measurements and calculation of the N2MBW indices. The means of the N2MBW indices from intentionally three and at least two technically acceptable measurements performed at each test occasion were reported in absolute values.1 Prior to implementing this technique in our setting, three operators completed a European standardization and certification process for measuring LCI through N2MBW with a commercial device (specific training and sharing of data for central over-reading).
Static and dynamic lung volumes were measured by body plethismography and spirometry (Jaeger MasterScreen Body, CareFusion, Hoechberg, Germany) after the N2MBW procedure. Absolute values for static lung volumes and z-scores for dynamic lung volumes and flows were recorded and performed according to the ERS/ATS standards.
Patient characteristics were presented by medians (ranges) or numbers and percentages of total. Patients with CF and PCD were grouped for comparison with healthy controls. Group differences were analysed using the independent t-test, Mann–Whitney U-test, and Chi-squared test as appropriate; p < 0.05 was accepted as statistically significant. Data analyses were performed using Microsoft Excel for Office 365 MSO and SPSS 24.0.
The study was approved by a local ethics community (Comissão de Ética para a Investigação Clínica). Informed consent for participation was obtained.
During the study period 34 children were assessed, five were excluded due to invalid MBW readings and two successfully repeated the procedure in a different testing session. The success rate for performing N2MBW for the first time was 75% (22 out of 29). Additionally, all healthy controls were naïve to lung function testing, and one was unable to perform spirometry properly.
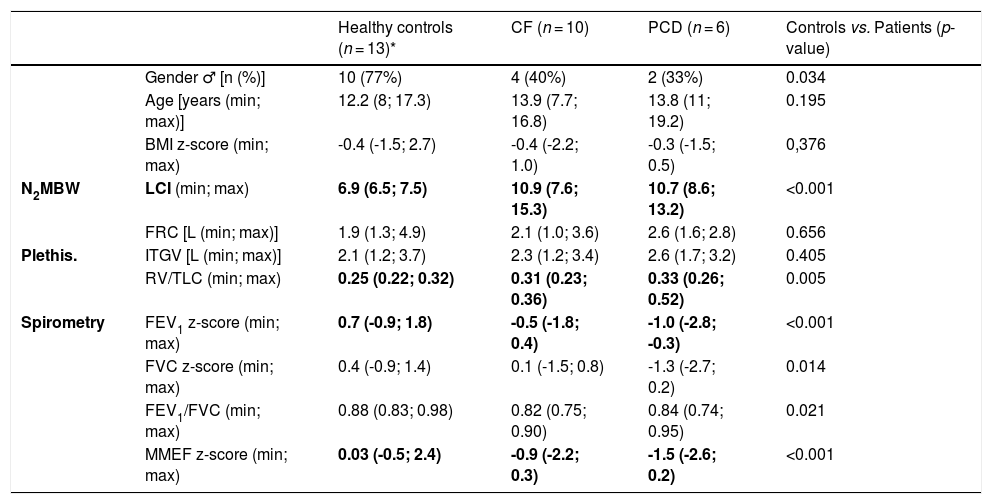
We analysed data from 29 children: ten CF, six PCD and thirteen healthy controls (Table 1). There were no differences between patients and healthy children regarding gender, age, or BMI. The diseased group had higher LCI [10.8 (7.6; 15.3) vs 6.9 (6.5; 7.5)], and lower FEV1 [-0.8(-2.8; 0.4) vs. 0.7(-0.9; 1.8)] and MMEF [-1.0 (-2.6; 0.3) vs. 0.03 (-0.5; 2.4)] when compared to healthy controls (p < 0.001).
Participants’ characteristics and lung function results.
| Healthy controls (n = 13)* | CF (n = 10) | PCD (n = 6) | Controls vs. Patients (p-value) | ||
|---|---|---|---|---|---|
| Gender ♂ [n (%)] | 10 (77%) | 4 (40%) | 2 (33%) | 0.034 | |
| Age [years (min; max)] | 12.2 (8; 17.3) | 13.9 (7.7; 16.8) | 13.8 (11; 19.2) | 0.195 | |
| BMI z-score (min; max) | -0.4 (-1.5; 2.7) | -0.4 (-2.2; 1.0) | -0.3 (-1.5; 0.5) | 0,376 | |
| N2MBW | LCI (min; max) | 6.9 (6.5; 7.5) | 10.9 (7.6; 15.3) | 10.7 (8.6; 13.2) | <0.001 |
| FRC [L (min; max)] | 1.9 (1.3; 4.9) | 2.1 (1.0; 3.6) | 2.6 (1.6; 2.8) | 0.656 | |
| Plethis. | ITGV [L (min; max)] | 2.1 (1.2; 3.7) | 2.3 (1.2; 3.4) | 2.6 (1.7; 3.2) | 0.405 |
| RV/TLC (min; max) | 0.25 (0.22; 0.32) | 0.31 (0.23; 0.36) | 0.33 (0.26; 0.52) | 0.005 | |
| Spirometry | FEV1 z-score (min; max) | 0.7 (-0.9; 1.8) | -0.5 (-1.8; 0.4) | -1.0 (-2.8; -0.3) | <0.001 |
| FVC z-score (min; max) | 0.4 (-0.9; 1.4) | 0.1 (-1.5; 0.8) | -1.3 (-2.7; 0.2) | 0.014 | |
| FEV1/FVC (min; max) | 0.88 (0.83; 0.98) | 0.82 (0.75; 0.90) | 0.84 (0.74; 0.95) | 0.021 | |
| MMEF z-score (min; max) | 0.03 (-0.5; 2.4) | -0.9 (-2.2; 0.3) | -1.5 (-2.6; 0.2) | <0.001 |
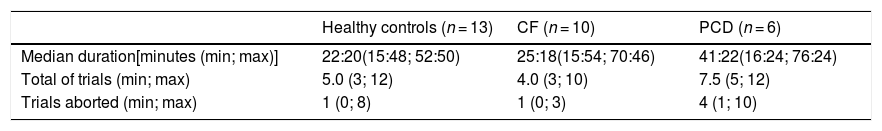
Executing MBW took 28 (16; 76) minutes [patient group 29 (16; 76) minutes; healthy controls 22 (16; 53 min], and it was attempted for 5 (3; 15) trials in each testing session (Table 2). For nine individuals (four healthy controls, two CF and three PCD), only two technically acceptable trials were achieved.
Nitrogen multiple breath washout feasibility.
CF: Cystic Fibrosis, PCD: Primary Ciliary Dyskinesia
We have shown that LCI is feasible with a high success rate on first attempt (75%) in school-age children, and throughout the first year of implementing N2MBW. Nevertheless, the procedure is time-consuming and largely dependent on specialized technicians.2 Also, N2MBW takes more time than routine spirometry and the time needed increases relative to the increase of LCI. In our setting, the duration of the test was influenced by the pathology and cooperation, and these challenges were concordant with guidelines and recommendations regarding this technique.1,5
Even though N2MBW tests were done as part of a research project, they were included in the patients’ clinic visits, allowing us to estimate its burden on routine lung function assessments, if we were to add this tool to the evaluation of children with chronic, progressive, obstructive lung disease.
In agreement with published data,6,7 we found that LCI was raised in children with CF and PCD compared to healthy controls, even in those with FEV1 within normal range. This is important as it shows that LCI in a clinical setting provided reliable data that reflects previous findings from studies in which the test had been undertaken in a research setting. Additionally, children with well-preserved spirometry, are those for whom monitoring with LCI will be most useful, and it is increasingly being adopted in CF centres for clinical decision making.2
Accordingly, anticipating the success rates within a clinical setting and assigning appropriate time slots for its inclusion is crucial when planning to implement this technique.
This brief, pragmatic report, provides information about the feasibility of N2BMW in children during routine visits, using the Exhalyzer®D.
There are no conflicts of interest
There was no financial support or sponsorship