Late-onset Pompe disease (LOPD) is an autosomal recessive disease caused by acid alpha–glucosidase deficiency. The phenotype is a progressive proximal myopathy. Respiratory failure is the main life-threatening complication, usually resulting from diaphragm weakness,1 which may be independent of the severity of motor involvement. Screening for diaphragm function include the assessment of postural drop in forced vital capacity (FVC) moving from sitting to supine position (ΔVC),2 and measurement of Maximal Inspiratory Pressure (MIP).
We previously observed by Magnetic Resonance Imaging (MRI) that axial muscles involvement (posterior trunk, abdominal wall) represents a feature peculiar to LOPD, otherwise uncommon in other myopathies. Axial involvement may be suspected in patients with chronic lumbar pain, hyper-lordosis, and abdominal prominence, but axial muscles are difficult to assess by clinical examination alone: thus, imaging fills a clinical need.3 What is the clinical impact, if any, of axial muscles involvement? Are there any functional correlates, beyond lumbar pain and postural changes? Indeed, trunk muscles may be involved in respiration, with posterior muscles participating in inspiration, and anterior abdominal wall muscles contributing to forced expiration.4 Our hypothesis is that axial involvement may be a sentinel sign of respiratory dysfunction, and that MRI of the axial muscles may represent an effective approach to screen for respiratory impairment in LOPD, to optimize pulmonary evaluation and treatment strategy for these individuals.
We investigated prospectively 19 patients (8 females) aged 54.6 ± 18.2 years (range 25–76) with genetically confirmed LOPD. Clinical, demographic, genetic data are in Supplementary Table 1.
Muscle MRI was performed as previously described3 by a 1.5T MRI scanner (1.5T Philips Intera and 1.5T Philips Ahieva XR Realeas) using T1-weighted spin-echo axial images from the mid-dorsal segment to the sacrum, using the same parameters (TR=300 ms, TE=10 ms, thickness =10 mm, matrix=640 × 640, in plane resolution 0.6 × 0.6 mm). Muscles were graded qualitatively according to the Mercuri score.3 We considered two muscles of the posterior wall of the lower trunk (Quadratus lumborum, Iliocostalis lumborum), and seven anterior wall muscles (Multifidus, Longissimus, Iliopsoas, Rectus abdominis, Transversus abdominis, Obliquus externus abdominis, Obliquus internus abdominis). Two independent observers blinded to clinical data examined all scans.
Respiratory assessment was performed within 48 h ofm MRI, according to standard guidelines.5 A postural drop of FVC (ΔVC) ≥30% was considered expression of diaphragmatic weakness;5 MIP was measured from the Functional Residual Capacity in the upright position; Maximal Expiratory Pressure (MEP) was measured at the Total Pulmonary Capacity. Both MIP and MEP were repeated at least three times or until two identical readings were obtained, with patients receiving strong verbal encouragement; the best value of both measurements was used.6
Deviations of quantitative variables from normality were calculated by the Shapiro-Wilk test (p<0.05). Quantitative variables with normal distribution are described as mean ± standard deviation or by median (25th–75th percentiles) otherwise. To test for significant differences in terms of normally distributed variables between binary conditions we used the Welch's t-test, and the Wilcoxon rank-sum test to test for differences in terms of variables deviation from the normal. Pairwise correlations were estimated by the Spearman test, associations between categorical variables by the Fisher's exact test. The significance threshold was set to p<0.008 based on the Bonferroni correction accounting for the number of muscles for which the MRI score was evaluated (α=0.05/6 tests). Univariate tests were applied to evaluate: a) significant correlations between MRI measurements and: MIP, MEP, FVC, ΔVC, FVC%; b) presence of significant associations between MRI measurements corresponding to the analyzed muscles and: Diaphragm ≥20 or Diaphragm ≥30. Statistical tests were performed by the R software v. 3.1.0 (www.r-project.org/).
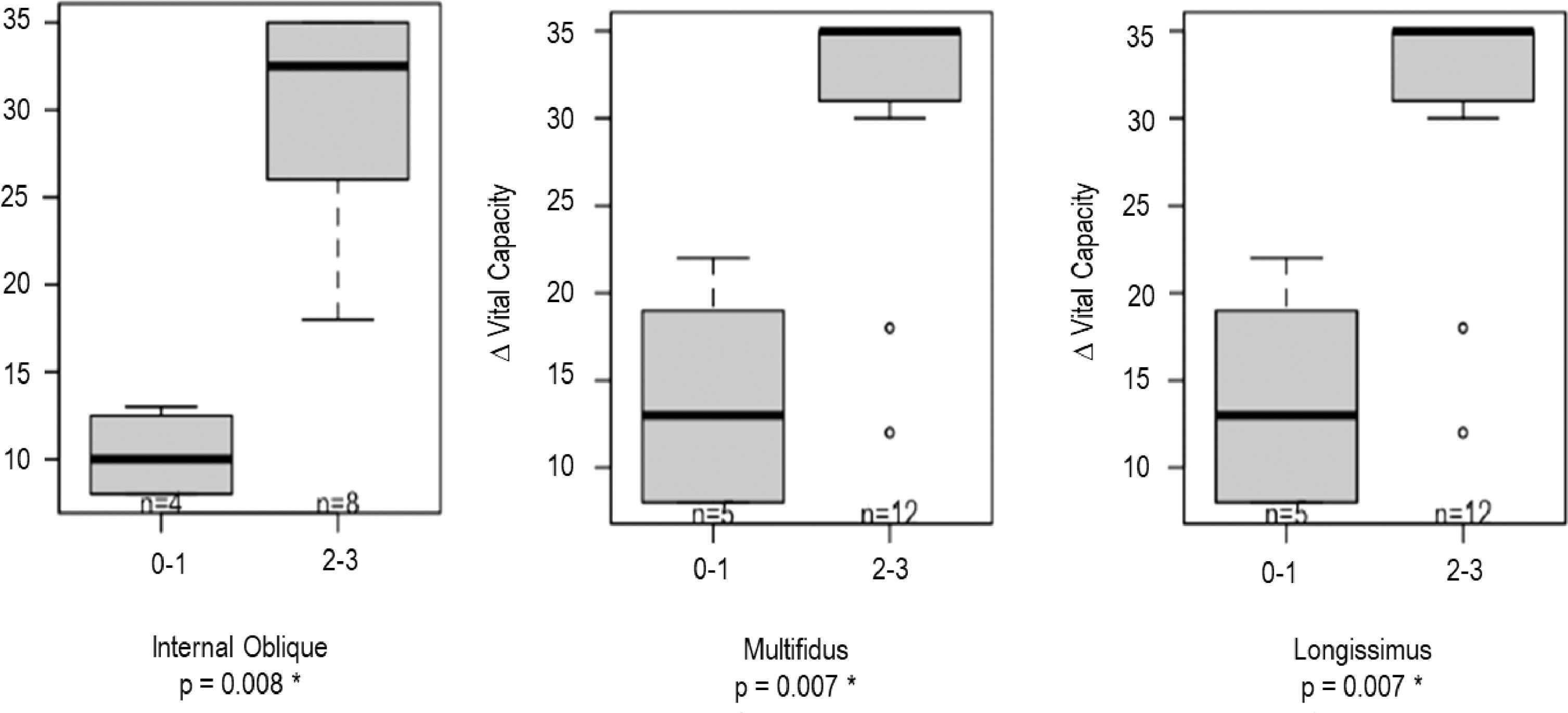
Summary statistics reporting the characteristics of the analyzed patients are in Supplementary Table 2 (quantitative variables) and 3 (categorical variables). Fig. 1 shows three different patterns of severity of trunk involvement. Involvement of the Internal Oblique and Multifidus correlated with worse MIP (rho=0.85, p = 0.004 and rho=0.75, p = 0.003 respectively). Similarly, Internal Oblique, Multifidus and Longissimus muscles were positively correlated with ΔVC values (rho=0.86, p<0.001; rho=0.80, p<0.001 and rho=0.73, p<0.001 respectively) (Fig. 2). Increased MRI scores for Multifidus were associated with increased probability of diaphragm ≥20 (p = 0.006) and diaphragm ≥30 (p = 0.005). Similarly, higher MRI scores for Longissimus were associated with increased probability of diaphragm ≥30 (p = 0.002). A weaker but still consistent correlation was found with Internal Oblique (p = 0.015).
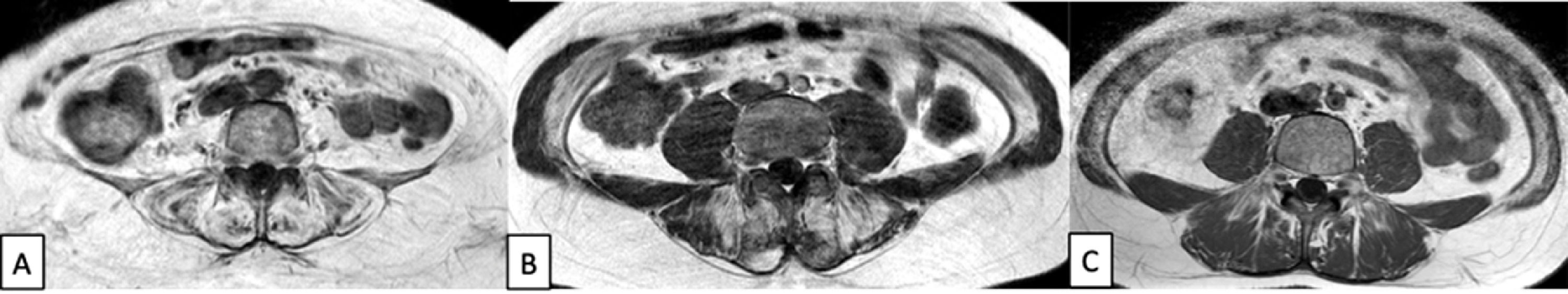
Muscle MRI with T1-w images at the level of the lumbar region showing three patients with different degrees of paraspinal muscle atrophy, in particular involving Quadratus Lumborum, Multifidus, Longissimus and Iliocostalis Lumborum. The first patient (A) shows a severe muscle atrophy, while the second (B) and the third (C) show respectively a moderate and a mild atrophy.
Internal Oblique, Multifidus and Longissimus muscles are positively correlated to ΔVC values. In particular, patients having MRI values ≥ 2 for these three parameters are characterized by a statistically significant increase in terms of ΔVC values with respect to those having MRI values ≤ 1 (p < 0.008).
Thus, posterior trunk atrophy was associated with decreased MIP, and both anterior and posterior trunk atrophy to postural drop; forced expiration (MEP) and upright FVC were not influenced by trunk muscles status. Indeed, posterior trunk muscles contribute to inspiration4 and are thus expected to influence postural drop and MIP.
The role of anterior/abdominal atrophy on postural drop may rather seem unexpected, given that abdominal muscles are essentially expiratory. We suggest that -in LOPD patients with diaphragm weakness- abdominal muscles may contribute to inspiration also, even during tidal breathing. At present, abdominal muscles contraction during expiration is conventionally regarded as beneficial to the act of breathing, because the consecutive rise in abdominal pressure induces diaphragm lengthening, placing diaphragm fibers on a more advantageous portion of their length-tension curve, and hence improving the force-generating ability of the diaphragm during the subsequent inspiration. Expiratory contraction of the abdominal muscles is a natural (automatic or spontaneous) component of the response of the normal respiratory system to greater than resting stimulation.7 When normal subjects increase their ventilation or breathe against inspiratory mechanical loads, they recruit the abdominal muscles, particularly the transversus, during expiration8: the associated reduction in end-expiratory lung volume allows the increased work of breathing to be shared between the inspiratory and the expiratory muscles. It is possible that in LOPD patients with diaphragm weakness, this "automatic" response to the imbalance of the inspiratory load/capacity relationship is already triggered during resting breathing, even though it may be useless and induce additional energy expenditure.
A limitation of our study is the lack of a control population. Further studies confirming our results are needed. Detection of axial involvement on MRI may be a warning sign of initial diaphragm weakness and respiratory dysfunction in clinostatism, since trunk weakness (and mainly abdominal weakness) is likely to impair the ability to compensate for diaphragmatic dysfunction in the supine position. Detection of trunk muscle damage by MRI may thus suggest the need of a closer respiratory follow-up or more extensive respiratory assessment, i.e. by polysomnography, even when upright FVC is still within normal ranges.
DeclarationsEthics approval and consent to participate:
The data collected are part of the regular follow-up of patients with Pompe disease. Data collection and consent to participate was approved by the Pavia Ethical Committee (IRCCS San Matteo Foundation), reference number p-20,160,022,743
Consent for publicationAll patients gave consent to collect their demographic and clinical data and to perform clinical and MRI investigations.
Availability of data and materialDatabase of clinical data is available to any scientist wishing to use them (Sabrina.ravaglia@mondino.it)
FundingThe work was supported by the Italian Ministry of Health funding, RC 2019; the funding body had no role in the study design and data collection and analysis.
Authors’ contributionThe Author's contributions are as follows: AC, SR, CD: methodology, conceptualization of results, manuscript writing and editing; NB: interpretation of physiological data; CD, PdF: genetic analysis; AM statistical analysis; SC: respiratory examinations and acquisition of data; AP: muscle MRI. All Authors read and approved the final manuscript.
Not applicable.