Clinical sequelae after COVID-19 have been well described, including abnormalities in pulmonary function tests, chest imaging, and patient-reported outcome measures.1,2 However, functional outcomes after COVID-19 are not well understood. We sought to identify the presence and underlying mechanisms of functional impairments after COVID-19. We hypothesized that patients with more severe COVID-19 would have a lower 6-minute walk distance (6MWD) at follow-up and that exertional dyspnea, fatigue, and hypoxemia would independently predict a lower 6MWD.
This was a consecutively-enrolled prospective cohort study. Patients who were seen in a hospital in Yucatan, Mexico with SARS-CoV-2 confirmed by real-time polymerase chain reaction were referred to a COVID-19 clinic for follow-up. Patients who were able to complete surveys, pulmonary function tests (PFTs), and 6-minute walk tests (6MWTs) were included. There were no exclusion criteria. PFTs and 6MWTs were conducted according to international guidelines.3–5 Patients did not receive formal physical rehabilitation during their recovery. This study received institutional ethics approval.
COVID-19 severity was categorized as mild (no hypoxemia), moderate (hypoxemia without mechanical ventilation), or severe (hypoxemia with mechanical ventilation). The association between COVID-19 severity and 6MWD was determined using multivariable linear regression, and underlying mechanisms for reduced 6MWD were then explored. Unadjusted and adjusted linear regression models were used to determine the association between potential predictor variables (Borg dyspnea, Borg fatigue, and end-exercise SpO2) and 6MWD, first in separate models and then in a final model with both Borg dyspnea and end-exercise SpO2 as co-primary endpoints to explore the independent relationship of these two predictors with 6MWD. All models were adjusted for age, sex, smoking, body mass index (BMI), and time from symptom onset. Statistical analyses were performed using R version 3.6.3 (R Foundation).
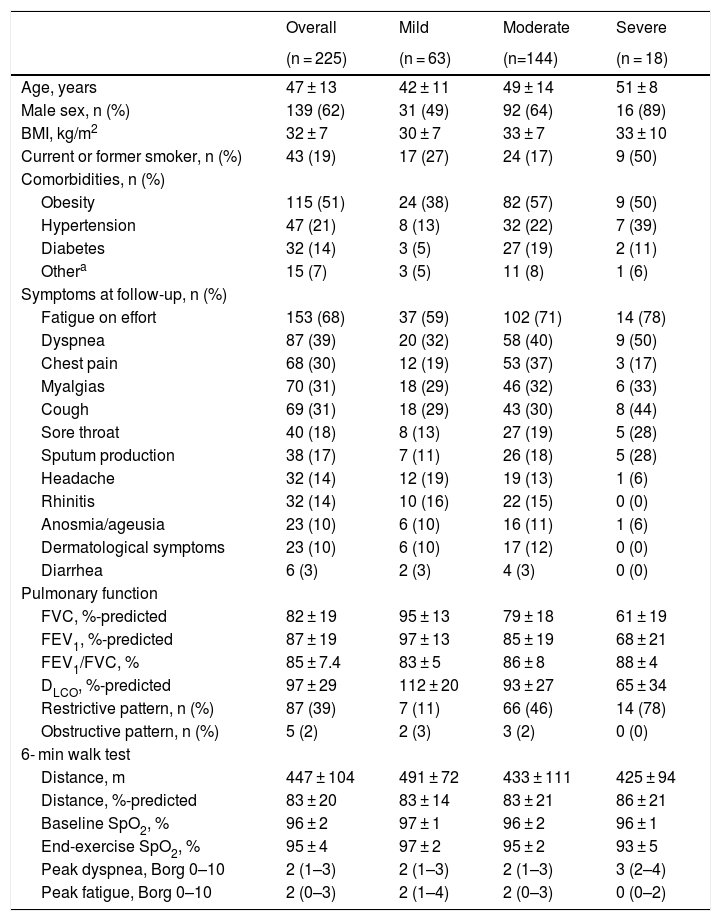
A total of 295 patients were referred to the COVID-19 clinic between May and August 2020, of whom 225 were enrolled (65 patients declined and 5 were lost to follow-up). The overall cohort had 62% males and 19% ever-smokers, with a mean age of 47 ± 13 years and BMI of 32 ± 7 kg/m2 (Table 1). There were 63 patients with mild, 144 with moderate, and 18 with severe COVID-19. The median follow-up time was 61 days (interquartile range [IQR] 50–75), with fatigue on effort (68%) and dyspnea (39%) being the most common symptoms.
Patient characteristics. Patients with mild (no hypoxemia), moderate (hypoxemia without mechanical ventilation), and severe (hypoxemia with mechanical ventilation) are shown. An obstructive pattern was defined as FEV1/FVC < LLN. A restrictive pattern was defined as normal FEV1/FVC with FVC-% predicted
| Overall | Mild | Moderate | Severe | |
|---|---|---|---|---|
| (n = 225) | (n = 63) | (n=144) | (n = 18) | |
| Age, years | 47 ± 13 | 42 ± 11 | 49 ± 14 | 51 ± 8 |
| Male sex, n (%) | 139 (62) | 31 (49) | 92 (64) | 16 (89) |
| BMI, kg/m2 | 32 ± 7 | 30 ± 7 | 33 ± 7 | 33 ± 10 |
| Current or former smoker, n (%) | 43 (19) | 17 (27) | 24 (17) | 9 (50) |
| Comorbidities, n (%) | ||||
| Obesity | 115 (51) | 24 (38) | 82 (57) | 9 (50) |
| Hypertension | 47 (21) | 8 (13) | 32 (22) | 7 (39) |
| Diabetes | 32 (14) | 3 (5) | 27 (19) | 2 (11) |
| Othera | 15 (7) | 3 (5) | 11 (8) | 1 (6) |
| Symptoms at follow-up, n (%) | ||||
| Fatigue on effort | 153 (68) | 37 (59) | 102 (71) | 14 (78) |
| Dyspnea | 87 (39) | 20 (32) | 58 (40) | 9 (50) |
| Chest pain | 68 (30) | 12 (19) | 53 (37) | 3 (17) |
| Myalgias | 70 (31) | 18 (29) | 46 (32) | 6 (33) |
| Cough | 69 (31) | 18 (29) | 43 (30) | 8 (44) |
| Sore throat | 40 (18) | 8 (13) | 27 (19) | 5 (28) |
| Sputum production | 38 (17) | 7 (11) | 26 (18) | 5 (28) |
| Headache | 32 (14) | 12 (19) | 19 (13) | 1 (6) |
| Rhinitis | 32 (14) | 10 (16) | 22 (15) | 0 (0) |
| Anosmia/ageusia | 23 (10) | 6 (10) | 16 (11) | 1 (6) |
| Dermatological symptoms | 23 (10) | 6 (10) | 17 (12) | 0 (0) |
| Diarrhea | 6 (3) | 2 (3) | 4 (3) | 0 (0) |
| Pulmonary function | ||||
| FVC, %-predicted | 82 ± 19 | 95 ± 13 | 79 ± 18 | 61 ± 19 |
| FEV1, %-predicted | 87 ± 19 | 97 ± 13 | 85 ± 19 | 68 ± 21 |
| FEV1/FVC, % | 85 ± 7.4 | 83 ± 5 | 86 ± 8 | 88 ± 4 |
| DLCO, %-predicted | 97 ± 29 | 112 ± 20 | 93 ± 27 | 65 ± 34 |
| Restrictive pattern, n (%) | 87 (39) | 7 (11) | 66 (46) | 14 (78) |
| Obstructive pattern, n (%) | 5 (2) | 2 (3) | 3 (2) | 0 (0) |
| 6- min walk test | ||||
| Distance, m | 447 ± 104 | 491 ± 72 | 433 ± 111 | 425 ± 94 |
| Distance, %-predicted | 83 ± 20 | 83 ± 14 | 83 ± 21 | 86 ± 21 |
| Baseline SpO2, % | 96 ± 2 | 97 ± 1 | 96 ± 2 | 96 ± 1 |
| End-exercise SpO2, % | 95 ± 4 | 97 ± 2 | 95 ± 2 | 93 ± 5 |
| Peak dyspnea, Borg 0–10 | 2 (1–3) | 2 (1–3) | 2 (1–3) | 3 (2–4) |
| Peak fatigue, Borg 0–10 | 2 (0–3) | 2 (1–4) | 2 (0–3) | 0 (0–2) |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; SpO2, oxygen saturation by pulse oximetry.
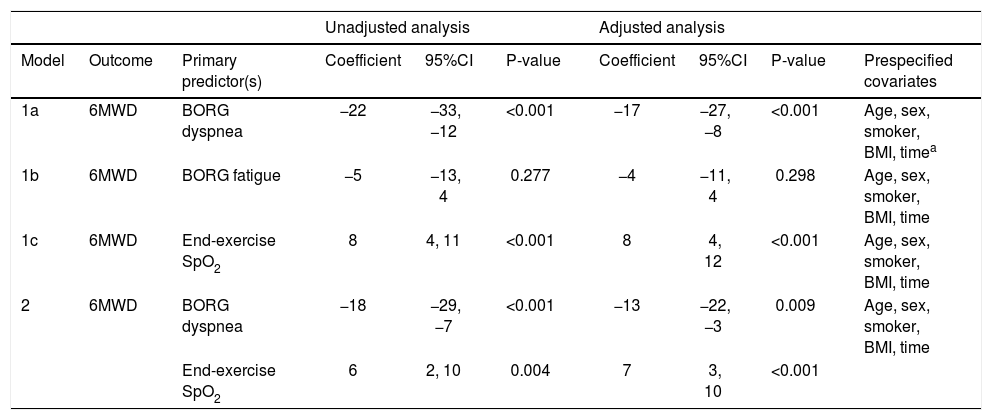
Patients with moderate or severe COVID-19 had a lower 6MWD compared to patients with mild disease (−51 m [95%CI −85, −17], p = 0.004 or −68 m [95%CI −134, −3], p = 0.04 respectively), with no difference between moderate and severe groups (p = 0.55). 6MWD was associated with both Borg dyspnea (coefficient −17 m per unit increase in Borg dyspnea [95%CI −27, −8]) and end-exercise SpO2 (coefficient 8 m [95%CI 4, 12]) (both p < 0.001). A sensitivity analysis using the delta SpO2 (i.e., end-exercise SpO2 — baseline SpO2) demonstrated similar results (coefficient 7 m per unit increase in delta SpO2 [95%CI 2, 13; p = 0.004]). Borg fatigue was not associated with 6MWD. When Borg dyspnea and end-exercise SpO2 were included as co-primary predictors in a single model, both variables remained independently associated with 6MWD with coefficients of −13 m (95%CI −22, −3) and 7 m (95%CI 3, 10), respectively, after adjusting for covariates (Table 2).
Mechanisms of reduced 6-min walk distance after COVID-19 illness.
| Unadjusted analysis | Adjusted analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Outcome | Primary predictor(s) | Coefficient | 95%CI | P-value | Coefficient | 95%CI | P-value | Prespecified covariates |
| 1a | 6MWD | BORG dyspnea | −22 | −33, −12 | <0.001 | −17 | −27, −8 | <0.001 | Age, sex, smoker, BMI, timea |
| 1b | 6MWD | BORG fatigue | −5 | −13, 4 | 0.277 | −4 | −11, 4 | 0.298 | Age, sex, smoker, BMI, time |
| 1c | 6MWD | End-exercise SpO2 | 8 | 4, 11 | <0.001 | 8 | 4, 12 | <0.001 | Age, sex, smoker, BMI, time |
| 2 | 6MWD | BORG dyspnea | −18 | −29, −7 | <0.001 | −13 | −22, −3 | 0.009 | Age, sex, smoker, BMI, time |
| End-exercise SpO2 | 6 | 2, 10 | 0.004 | 7 | 3, 10 | <0.001 | |||
Abbreviations: 6MWD, 6-min walk distance; BMI, body mass index; CI, confidence interval, SpO2, oxygen saturation by pulse oximetry.
A lower 6MWD was independently associated with exertional dyspnea and hypoxemia, suggesting that dyspnea and hypoxemia may have distinct mechanisms through which they impact functional capacity. Of the patients who had exertional hypoxemia (i.e., SpO2 decline ≥4%), 45% had a walk distance less than the lower limits of normal (LLN) and 100% had a DLCO < LLN, which suggests that desaturation during exercise is associated with parenchymal and/or pulmonary vascular phenomena. Although dyspnea is typically accompanied by hypoxemia during acute COVID-19 illness,6 our study found that exertional dyspnea predicted reduced functional capacity, regardless of whether end-exercise hypoxemia was present or not. The underlying mechanisms of persistent dyspnea after COVID-19 remain unclear; however, it is likely that physiologic sequelae contribute to this lingering symptom. In a previous study using the same cohort, we demonstrated that patients with persistent dyspnea had lower FVC, forced expiratory volume in 1 s, and higher proportion of restrictive ventilatory pattern compared to patients without persistent dyspnea.6 Furthermore, patients with abnormal DLCO at follow-up are more likely to have an elevated d-dimer on admission, suggesting that microangiopathies could contribute to dyspnea.7
This study had several limitations. First, our data did not include validated tools such as the Charlson Comorbidity Index to assess how comorbidities impact 6MWD. Second, this study was from a single Mexican center. However, this unique population adds to the understanding of COVID-19 recovery in diverse patient backgrounds. Third, we did not have information on treatment during the acute illness which could impact outcomes.
We demonstrate the impact that persistent dyspnea and hypoxemia have on functional capacity in patients after COVID-19. Further research to understand the underlying mechanisms of persistent symptoms, particularly dyspnea that is disproportionate to physiologic and radiologic findings, is needed in order to help patients recovering from COVID-19.
FundingThe authors received no funding for this work.
Conflict of interestThe authors have no conflicts of interest to declare.