The COVID-19 pandemic originated in China and within about 4 months affected individuals all over the world. One of the limitations to the management of the COVID-19 is the diagnostic imaging to evaluate lung impairment and the patients’ clinical evolution, mainly, in more severe cases that require admission into the intensive care unit. Among image examinations, lung ultrasound (LU) might be a useful tool to employ in the treatment of such patients.
MethodsA survey was carried out on PubMed to locate studies using the descriptors: ((Lung ultrasound OR ultrasound OR lung ultrasonography OR lung US) AND (coronavirus disease-19 OR coronavirus disease OR corona virus OR COVID-19 OR COVID19 OR SARS-CoV-2)). The period covered by the search was November 2019 to October 2020 and the papers selected reported LU in COVID-19.
ResultsForty-three studies were selected to produce this systematic review. The main LU findings referred to the presence of focal, multifocal and/or confluent B lines and the presence of pleural irregularities.
ConclusionsThe use of LU in the evaluation of patients with COVID-19 should be encouraged due to its intrinsic characteristics; a low cost, radiation free, practical method, with easy to sanitize equipment, which facilitates structural evaluation of lung damage caused by SARS-CoV-2. With the increase in the number of studies and the use of ultrasound scans, LU has been shown as a useful tool to evaluate progression, therapeutic response and follow-up of pulmonary disease in the patients with COVID-19.
COVID-19 (Coronavirus Disease 2019) is an illness caused by the SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) that mainly affects the breathing system.1 Last year COVID-19 affected individuals all over the world. In total, over 98 million cases of the disease and 2 million deaths have been recorded.2,3 The SARS-CoV-2 presents a 7% case fatality rate globally, however, it varies greatly in different countries of the world. The death rate of COVID-19 is not the best marker to estimate the severity of the illness, since its diagnosis is based on the Real Time Polymerase Chain Reaction (RT-PCR) and in many countries, like Brazil, the access to this resource is reduced due to lack of materials, equipment, transport logistics and laboratory staff to meet the demand for exams.4,5 Thus, many SARS-CoV-2 colonization asymptomatic cases or those presenting mild severity are not recorded by the health system.
The COVID-19 became a global challenge in 2020 due to the SARS-CoV-2 high virulence and the fact that it has been able to cross borders and reach populations all over the world, causing high demand on health services and the need for the action of multidisciplinary teams throughout the pandemic.5 Management of the disease requires the use of intensive care units; however, not all countries have been able to deal with the high number of individuals affected simultaneously and there has been a collapse in the health service of many countries that has resulted in the need for political interventions.6 In the global context, social distancing has been described as the most efficient mechanism for reducing virus spread and controlling the COVID-19 pandemic.7–9
The COVID-19 clinical variability is mainly dependent on underlying diseases and age.10 The literature reports alterations of the pulmonary function, and the use of lung high resolution computed tomography (HRCT) is advised to determine the extent of the damage.11 However, this examination presents high cost, difficult accessibility for the patients affected, necessity of physical structure and patient transportation to the tomography equipment, exposure to radiation and lack of applicability during hospitalization. Thus, among the image exams, the lung ultrasound (LU) stands out and might become a useful tool for use in the treatment and follow up of patients with COVID-19, mainly in more severe cases when intensive care is required. In this context, we carried out this systematic review of the use of LU in COVID-19 in 2019 and 2020.
MethodsThe systematic review was carried out using the data base PubMed/Medline and according to the preferred reporting items for systematic review and meta-analysis (PRISMA) covering the period from December 2019 to October 2020. The following descriptors guided the search: ((Lung ultrasound OR ultrasound OR lung ultrasonography OR lung US) AND (coronavirus disease-19 OR coronavirus disease OR corona virus OR COVID-19 OR COVID19 OR SARS-CoV-2)) with the following filters: clinical study; clinical trial; clinical trial protocol; clinical trial, phase I; clinical trial, phase II; clinical trial, phase III; clinical trial, phase IV; comparative study; controlled clinical trial; guideline; journal article; observational study; practice guideline; and randomized controlled trial. Also, the filter for Humans and English language were used.
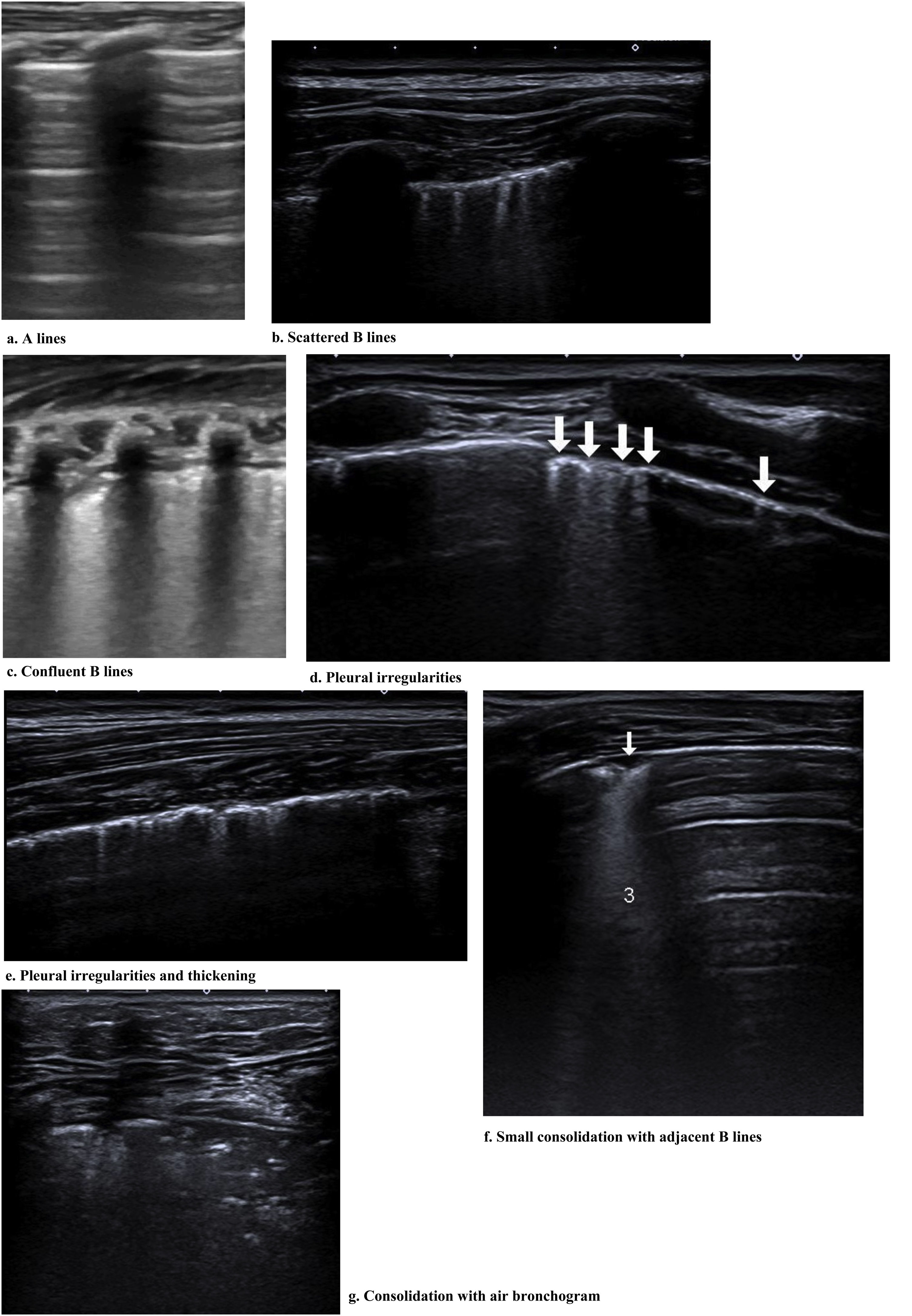
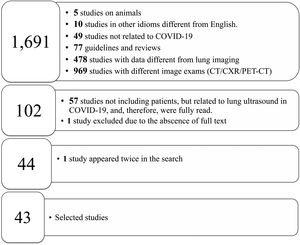
From the 1,691 studies found, the papers excluded were: (i) 10 in other languages (8 published in Chinese and 2 in Spanish); (ii) 77 reviews and guidelines; (iii) 969 papers that addressed other image exams such as HRCT, positron emission tomography combined with computed tomography and thorax radiography without the use of LU; (iv) 483 due to the approach of different themes that were related to LU or those that had been developed using animals; (v) 77 guidelines and systematic reviews without association with LU (Fig. 1).
The systematic review flowchart. The systematic review was carried out using the data base PubMed/Medline and according to the preferred reporting items for systematic review and meta-analysis (PRISMA) covering the period from November 2019 to October 2020. The following descriptors guided the search: ((Lung ultrasound OR ultrasound OR lung ultrasonography OR lung US) AND (coronavirus disease-19 OR coronavirus disease OR corona virus OR COVID-19 OR COVID19 OR SARS-CoV-2)). LU = lung ultrasound; CT = computed tomography; CXR = chest X-ray; PET-CT = positron emission tomography combined with computed tomography; COVID-19 = coronavirus disease-2019.
A total of 102 papers were evaluated, at least title and abstract, and some of these reports were selected to support this paper’s introduction and discussion and provide some theoretical-scientific basis of LU, from those, 57 addressed the LU role in COVID-19 without evaluating patients with that disease. Consequently, 45 papers were selected for the literature review. The inclusion criterion was the search for references to the application of LU in the investigation of SARS-CoV-2 infection. Three authors (AOP, RMC and RU) selected the titles and abstracts of the papers individually and there was no disagreement regarding this choice of material to produce the systematic review. Out of the 45 papers, one was excluded for lack of access to the full text even after contacting the corresponding author and one was dismissed because it appeared twice in the search, that is, the same study was published in the same journal, once as a short communication and another time as a letter to the editor.
The data collected from each study were: author (year of publication), type of study, number of patients with COVID-19 or number of participants, COVID-19 diagnostic method, clinical characteristics of the patients, LU findings, use of other imaging exams, scanning areas/LU technique/sort of equipment, patient treatment, the use of individual protection equipment by the professional carrying out the LU, machine cleaning and comments.
ResultsForty-three studies reported lung impairment in COVID-19 evaluated by the LU propaedeutic. Those studies totaled 2,116 patients, including children, adults, elderly and pregnant women, 863 male and 1,210 female individuals, and 43 patients whose gender was not given, the age of the patients ranged from 0 to 106 years. The country with the highest number of reports on the use of this clinical tool was Italy with 15 studies,12,15,25,27,30,31,34,64,69,71,77,78,82,86,87 followed by China with 8 studies,29,38,62,67,70,73,76,83 Spain with 6 studies,35,72,79,80,85,89 France with 5 studies,32,33,65,68,88 Canada with 2 studies,18,19 Brazil,63 Bhutan81 Germany,84 Israel,75 South Korea,74 Turkey66 and USA26 with 1 study each.
The results of the characteristics of the studies investigating LU in COVID-19 are presented in Tables 1 and 2.
Clinical characteristics of studies evaluating lung ultrasound in COVID-19.
| Study | Country | Type of study | Patients and participants included in the studies | Diagnosis of COVID-19 | Clinical picture | Comorbidities | Treatment |
|---|---|---|---|---|---|---|---|
| Thomas et al.19 | Canada | Case report | 64 years old, female and health professional. | RT-PCR | Productive cough and dyspnea on exertion. After 6 days in O2 catheter → invasive mechanical ventilaton. 88% SpO2. | NS | Support: Invasive mechanical ventilation + intubation. |
| Soldati et al.12 | Italy | Protocol | 30 patients. | NS | NS | NS | NS |
| Buonsenso et al.15 | Italy | Case report | 1 adult, 52 years old, male. | NS | Fever, asthenia, cough, headache, myalgia, photophobia for 1 week; 90% SpO2. Dyspnea and bilateral rales. | NS | NS |
| Kim et al.18 | Canada | Case report | 1 man, 67 years old. | NS | Fever and chills for 5 days, non-productive cough, myalgia and malaise. 80/40 arterial pressure, 38.7oC temperature, 120 cardiac rate, 24 RF, 93% SpO2 in ambient air. | Hypertension and dyslipidemia. | NS |
| Denina et al.25 | Italy | Descriptive observational | 8 children and adolescents (0–17 years old), divided into 3 female and 5 male participants. | NS | Fever (6 patients); dry cough (5 patients); dyspnea/tachypnea (3 patients); odynophagia (3 patients); vomit or diarrhea (3 patients) and hypoxemia (2 patients). | NS | Oxygen therapy. |
| Yasukawa et al.26 | USA | Analytical observational | 10 adults (31–71 years old), divided into 7 male and 3 female participants. | Detection of SARS-CoV-2 in nasopharyngeal swab RT-PCR. | Fever, cough, dyspnea, SpO2 from 89 to 96%. | Rheumatoid arthritis, SAH, asthma, sleep obstructive apnea, obesity, hyperlipidemia and atrial fibrillation. | Oxygen therapy with mask (4 patients). |
| Musolino et al.27 | Italy | Analytical observational | 10 children (mean age 11 years). | Nasopharyngeal swab RT-PCR. | Fever (80%), cough (50%), anosmia (10%), arthralgia (30%), chest pain (20%), headache (20%). | NS | Patient did not require hospital treatment or ICU. |
| Ji et al.29 | China | Case report | 1 female adult (60 years old). | Oropharyngeal swab RT-PCR. | Fever, chills, dry cough, fatigue and dyspnea. RF 30 breaths per minute; 92% SpO2 in ambient air. | SAH and systemic lupus erythematosus. | Respiratory support and interferon inhalation. |
| Buosenso et al.30 | Italy | Case report | 4 pregnant women (31–42 years old — mean age 37 years). Gestacional periods=17, 24, 35 and 38 weeks. | Nasopharyngeal swab RT-PCR. | NS | No comorbidities. | All patients received hydroxychloroquine, lopinavir/ritonavir, no need for ICU. Tocilizumab was added for the patient based on the pulmonary impairment revealed by the LU. |
| Inchingolo et al.31 | Italy | Descriptive observational | 1 pregnant woman (age not informed). Gestational period = 23 weeks. | Oropharyngeal swab RT-PCR. | Cough and fever, eupneic, no respiratory discomfort, 98% SpO2 in ambient air. Bilateral reduced vesicular murmur in bases. | NS | NS |
| Duclos et al.32 | France | Case report | 1 male adult. | Nasopharyngeal swab RT-PCR. | Dry cough (4 patients); anosmia (1 patient); fever ≥38 °C (3 patients) temperature. | NS | NS |
| Zieleskiewicz et al.33 | France | Case report | 2 older people (65-year-old male; and 72-year-old female participants). | NS | NS | NS | Mechanical ventilation for the 65-year-old patient. |
| Youssef et al.34 | Italy | Case report/Letter to the editor | 1 pregnant woman, 33 years old, gestational period = 26 weeks. | Positive nasopharyngeal swab. | Fever, mild chest pain and dyspnea for three days, with normal oxygen saturation. | NS | NS |
| Tung-Chen et al.35 | Spain | Case report | 35-year-old male adult. | RT-PCR | Abrupt chills and sickness, dry cough after 20 h of isolation, bilateral cephalgia and normal lung auscultation. | NS | Supportive therapy was started with ibuprofen and paracetamol. After confirming worsening of symptoms and LU findings, hydroxychloroquine 200mg twice a day and azithromycin were added to the treatment. |
| Lu et al. 38 | China | Observational | 30 (16 male and 14 female) with mean age of 52 ± 15 years. | RT-PCR — including two patients with positive results. | Fever: 20 patients (66.7%); cough: 14 patients (46.7%); fatigue: 5 patients (16.7%); muscle soreness: 5 patients (16.7%); nausea: 2 patients (6.7%); no obvious symptoms: 3 patients (10%). | NS | NS |
| Tan et al.62 | China | Case series | 12 (4 male and 8 female), ranging from 52 to 79 years old, mean age 60.5 years. | RT-PCR | Moderate (4 patients): fever, diarrhea or other respiratory tract symptoms.Severe (4 patients) — showing any of the following: RF ≥30 times/min OR at rest, peripheral venous oxygen saturation ≤93% or PaO2/FiO2 ≤300 mmHg.Critical (4 patients) — showing any of the following: respiratory failure with mechanical ventilation OR shock OR with other organic failure and need for admission in the ICU. | Hypertension (1 patient), diabetes mellitus (1 patient) and cardiovascular diseases (2 patients). | NS |
| Mafort et al.63 | Brazil | Transversal observational | 409 (134 male and 275 female), ranging from 35 to 51 years old (mean age 41 years) — all of them health care professionals. | RT-PCR | Cough (84%); fever (69.7%); dyspnea (36.2%). | NS | NS |
| Veronese et al.64 | Italy | Retrospective study | 48 patients living in nursing homes (women = 81.3%), mean age 84.1 years. | RT-PCR | NS | Dementia and mostly bedridden patients. | NS |
| Zieleskiewicz et al.65 | France | Observational study with retrospective analysis | 100 (65 male and 35 female), ranging from 54 to 77 years old (mean age 61 years). | RT-PCR | Acute dyspnea (SpO2 <94% or breathing difficulty). | Body mass index >30 (17%); SAH (24%); coronariopathy (11%); cardiac failure (16%); diabetes mellitus (16%); chronic obstructive pulmonary disease (10%); cancer (7%); chronic kidney disease (2%), hepatopathy (1%) and immunosuppression (1%). | NS |
| Yassa et al.66 | Turkey | Prospective cohort | 296 pregnant women (23 with positive result for COVID-19), age range from 17 to 43 years old (mean age 26.8 years); gestacional period from 5 to 42 weeks (mean gestacional period 35.18 weeks). | RT-PCR | Pregnant women admitted in Gynecology and Obstetrics unit for any reason were tested for SARS-CoV-2 RT-PCR and examined with LU; 23 pregnant women with positive SARS-CoV-2 RT-PCR result, from whom 11 (3.72%) were symptomatic and 12 (4.05%) were asymptomatic. | NS | NS |
| Zhao et al.67 | China | Retrospective study | 35 (24 men and 11 women) patients divided into 2 groups: refractory (7 patients), mean age 62.14 years and non-refractory (28 patients), mean age 64.14 years. | RT-PCR | 1. Severe: respiratory distress with RF ≥30, SpO2 ≤93% and PaO2/FiO2 ≤300 mmHg, at rest. Non-refractory.2. Critical: respiratory failure requiring mechanical ventilation, shock and another organic failure requiring admission in the ICU. Non-refractory.3. Refractory: refractory respiratory disease with PaO2/FiO2 ≤100 mmHg or patients treated with ECMO. | NS | High flow nasal cannula; mechanical ventilation; ECMO. |
| Dargent et al.68 | France | Prospective study | 10 (8 men and 2 women) ages ranging from 46 to 63 (mean age 56 years). | RT-PCR | Moderate to severe ARDS. | Obesity | Mechanical ventilation. |
| Bonadia et al.69 | Italy | Prospective cohort | 41 (28 men and 13 women) mean age 60 ± 22.7 years. | RT-PCR | 24 patients (58.5%) with dyspnea; 32 patients (78%) with fever; 27 patients (65.8%) with cough. | NS | Ventilatory support: none in 11 (26.8%); low flow oxygen in 13 (31.7%); high flow oxygen in 2 (4.9%); non-invasive positive pressure ventilation in 9 (21.9%); intubation in 6 (14.6%). |
| Deng et al.70 | China | Retrospective study | 128* (75 men and 53 women) ages ranging from 55 to 71 years old (mean age 65 years). | RT-PCR | Divided into 4 groups:1. Light: light symptoms without HRCT alteration.2. Common: fever and signals of respiratory infection with pneumonia alterations in the HRCT.3. Severe: any of these symptoms, (a) respiratory distress with RF ≥30, (b) SpO2 ≤93% at rest or (c) PaO2/FiO2 ≤300 mmHg.4. Critical: (a) respiratory failure requiring mechanical ventilation, (b) shock, (c) admission in the ICU due to multiple organ failure. | 44 (34%) patients with hypertension; 22 (17.2%) patients with coronary disease; 19 (14,8%) patients with diabetes melittus; fatigue (96.1%); fever (95.3%) and breathlessness (94.5%); decreased SpO2 in 99 (77.3%) patients. | Oxygen therapy in all patients. Non-invasive ventilation in 38 patients; mechanical ventilation in 31 patients; ECMO in 4 patients; and 42 patients in the ICU. * Out of the 128 participants, 7 remained in hospital, 84 were discharged and 37 died. |
| Pagano et al.71 | Italy | Observational study | 18 (13 men and 5 women), mean age 69 years. | RT-PCR | Light to moderate ARDS. | NS | Non-invasive CPAP. |
| Martinez et al.72 | Spain | Case series | 3 (pediatrics age range without specifying the individuals’ ages). | RT-PCR | NS | Severe, but not specific. | NS |
| Yu et al.73 | China | Case report | Case 1 = 54-year-old man. | RT-PCR | Case 1 = cough. | NS | NS |
| Case 2 = 37-year-old woman. | RT-PCR | Case 2 = tightness in chest for a week, solved at admission. Without respiratory symptoms. | NS | NS | |||
| Cho et al.74 | South Korea | Case series | 6 (2 men and 4 women) ages ranging from 16 months to 85 years old. | RT-PCR | Case 1 = sore throat, backache, dry cough and fever on the 5th day. | NS | Case 1 = lopinavir/ritonavir. |
| RT-PCR | Case 2 = cough and chills for a day, fever >37.5 °C temperature and myalgia. | NS | Case 2 = lopinavir/ritonavir. | ||||
| RT-PCR | Case 3 = dyspnea and fever >37.5 °C temperature. | NS | Case 3 = OTI and mechanical ventilation. | ||||
| RT-PCR | Case 4 = fever for 8 days, dyspnea. | NS | Case 4 = empirical antibiotic therapy and oseltamivir followed by lopinavir/ritonavir; OTI, methylprednisolone + inhaled nitric oxide and veno-venous ECMO. | ||||
| RT-PCR | Case 5 = rhinorrhea, nasal obstruction and sputum. | NS | Case 5 = NS | ||||
| RT-PCR | Case 6 = asymptomatic and stable. | NS | Case 6 = no need for treatment. | ||||
| Lichter et al.75 | Israel | Prospective study | 120 (74 men and 46 women) mean age 64.7 ± 18.2 years. | RT-PCR | Respiratory symptoms; fever; chest pain; fatigue; SpO2 with 95% median and 89–98% interval. | Found in 81% of the patients: hypertension in 67 (55.8%); diabetes mellitus in 34 (28.3); obesity (not informed %); atrial fibrilation/flutter in 21 (17.5%); ischemic cardiac disease in 21 (17.5%); transient ischemic attack/stroke in 14 (11.7%). | NS |
| Lu et al.76 | China | Observational study | 16 (9 men and 7 women), ages ranging from 47 to 68 years old (mean age 58 years). | NS | Severe COVID-19 consistent with any of the following criteria: respiratory difficulty, RF >30 or SpO2 <93% in ambient air or PaO2/FiO2 ≤300 mmHg or pulmonary lesion with over 50% progression in 24-48 h in imaging examination. | NS | High flow cannula, non-invasive ventilation and OTI with mechanical ventilation. |
| Dini et al.77 | Italy | Observational study | 150 (23 men and 127 women), ages ranging from 72 to 106 years old (mean age 88 years). | RT-PCR | Respiratory symptoms, cough, dyspnea, fever, asthenia. | 92 (61.3%) patients with hypertension; 35 (23%) patients with chronic kidney disease; 28 (18.7%) patients with diabetes mellitus; 25 (16.7%) patients with coronary disease; 41 (27.3%) patients with other cardiac diseases; 44 (29.3%) patients with stroke; 28 (18.7%) patients with atrial fibrillation; 12 (8%) patients with cardiac failure; chronic obstructive pulmonary disease in 13 (8.7%) patients. | NS |
| Iodice et al.78 | Italy | Observational study | 29 (26 men and 3 women), ages ranging from 34 to 79 years old (mean age 60 years). | RT-PCR | Fever: 26 (90%) patients; cough: 15 (52%) patients; dyspnea: 8 (28%) patients; arthralgia: 4 (14%) patients; conjunctivitis: 2 (7%) patients. | 15 (62%) patients with hypertension; 5 (21%) patients with diabetes mellitus; 4 (17%) patients with asthma; 6 (21%) smoker patients. | Oxygen therapy in 23 (79%) patients. |
| Tung-Chen et al.79 | Spain | Prospective study | 51 (28 men and 23 women), mean age 61.4 years. | RT-PCR | Dyspnea: 29 (56.9%) patients; fever: 23 (45.1%) patients; myasthenia: 22 (43.1%) patients; gastrointestinal tract symptoms: 10 (19.6%) patients; cough: 22 (43.1%) patients; ageusia/anosmia: 4 (7.8%) patients. | 14 (27.5%) patients with cardiovascular disease; 12 (23.5%) patients with pulmonary disease; 10 (19.6%) patients with diabetes mellitus; 6 (11.8%) patients with chronic kidney disease; 8 (15.8%) patients with immunosuppression; 20 (39.2%) patients with hypertension; 13 (25.5%) patients with malignity. | NS |
| Gregorio-Hernández et al.80 | Spain | Case report | Case 1 (male newborn)= 2 days old and gestacional period = 38 + 3 | RT-PCR | Case 1 = mother with postpartum fever without respiratory symptoms. | Case 1: meconium aspiration syndrome. | Case 1: mechanical ventilation, nitric oxide, vasoactive drugs, cooling therapy and anticonvulsants (due to severe hypoxic ischemic encephalopathy). |
| Case 2 (male newborn)=78 days old and gestacional period=39+3. | RT-PCR | Case 2 = asymptomatic, but investigated after case 1 diagnosis. | Case 2: prematurity and bronchopulmonary dysplasia. | Case 2: oxygen therapy. | |||
| Case 3 (male newborn) = 6 days old and gestacional period=39+6 (gestacional period at the moment of the diagnosis). | RT-PCR | Case 3=asymptomatic, but investigated after case 1 diagnosis. | Case 3: Hirschsprung. | Case 3: no need for respiratory support. | |||
| LeVine et al.81 | Bhutan | Case report | A 76-year-old man. | RT-PCR | Swell, loss of appetite, diarrhea and fatigue in the first 48 h. Cough and dyspnea with 78% SpO2 in ambient air on the 4th day of symptoms. | Hypertension, hyperlipidemia, neuropathy and splenectomy due to mantle cell lymphoma. | Oxygen therapy, OTI and prone position, intravenous immunoglobulin, oseltamivir, ceftriaxone, doxycycline, lopinavir/ritonavir and antibiotic substitution with meropenem and vancomycin. |
| Nouvenne et al.82 | Italy | Observational study | 83 participants (23 men and 60 women), mean age 85 ± 8 years. | RT-PCR | 33 (40%) patients with cough; 52 (63%) patients with fever; 33 (40%) patients with dyspnea or light desaturation. | NS | Empirical pharmacological treatment with antibiotics, hydroxychloroquine and corticosteroids. |
| Yang et al.83 | China | Observational study | 29 participants (18 men and 11 women), mean age 55.2 ± 16 years. | NS | NS | NS | NS |
| Schmid et al.84 | Germany | Case report | A 76-year-old man. | NS | Fever for four days; dry cough and diarrhea; tachypnea, respiratory failure, 93% SpO2 (with O2 15 L/min in mask). | Absence of comorbidities. | Intensive treatment, not specified. |
| López Zúñiga et al.85 | Spain | Case report | Case 1 = 87-year-old man. | Cases 1 = positive RT-PCR. | Case 1 = dyspnea, dry cough, no fever. | NS | NS |
| Case 2 = 53-year-old man. | Case 2 = negative RT-PCR with positive serology. | Case 2 = fever, cough, dyspnea. | |||||
| Case 3= 55-year-old man. | Case 3 = positive RT-PCR. | Case 3 = dyspnea. | |||||
| Case 4 = 35-year-old-man. | Case 4=diagnositc exam not specified. | Case 4 = fever for 3 days. | |||||
| Giacomelli et al.86 | Italy | Case report | A 67-year-old man. | RT-PCR | Fever for 7 days, absence of cough or dyspnea; 89% SpO2 in ambient air. | Hypertension; surgical background of abdominal aorta aneurysm open repair with graft in 2014. | Antiviral therapy (lopinavir/ritonavir); hydroxychloroquine; thrombotic prophylaxis with prophylactic subcutaneous enoxaparin; CPAP and introduction of methylprednisolone and tocilizumab; OTI and prone position. After worsening and evidence of the abdominal aorta graft thrombosis, introduction of sodium heparin and the use of vasoactive drugs. |
| Nouvenne et al.87 | Italy | Transversal observational | 26 participants (14 men and 12 women), mean age 64 ± 16 years. | RT-PCR | 25 (96%) patients with fever; 21 (81%) patients with cough; 10 (38%) patients with dyspnea. | Comorbidities in 19 (73%) patients, but not specified. | Oxygen therapy in 17 (26%) patients. |
| Peyrony et al.88 | France | Prospective observational cohort | 391 participants (241 men and 150 women) ages ranging from 48 to 71 years old (mean age 62 years). | Positive RT-PCR in 225 (57.6%) patients. | Fever in 176 (78.2%) patients; cough in 158 (70.2%) patients; dyspnea in 131 (58.2%) patients; myalgia in 71 (31.6%) patients; rhinitis/pharyngitis in 19 (8.4%) patients; anosmia in 31 (13.8%) patients. | Immunosuppression in 195 (50.5%) patients. Chronic pulmonary disease in 85 (22.1%) patients. Cardiovascular disease in 156 (40.4%) patients. Obesity in 58 (15.2%) participants. | NS |
| Rodriguez-Gonzalez et al.89 | Spain | Case report | A 6-month-old male participant. | Negative RT-PCR. Detection of anti-SARS-CoV-2 Immunoglobulin M and anti-SARS-CoV-2 Immunoglobulin G on day 21 of illness. | 2-week history of nasal congestion and cough, irritability, tachypnoea (80 breaths per minute), cyanosis (81% SpO2), tachycardia (170 beats per minute), hypotension (59/32 mmHg), poor perfusion, weak peripheral pulses and hepatomegaly (3 cm). | Short bowel syndrome with fever and cyanosis; cardiogenic shock secondary to severe pulmonary hypertension and right ventricular failure without pulmonary thromboembolism condition labelled as pediatric multisystem inflammatory syndrome. | Prophylaxis with low molecular weight heparin; mechanical ventilation and prone position; inotropic support with milrinone and norepinephrine and broad-spectrum antibiotics (meropenem, vancomycin and fluconazole); Tocilizumab, azithromycin, hydroxychloroquine and methylprednisolone. |
COVID-19 = coronavirus disease 2019; RT-PCR = real time polymerase chain reaction; O2 = oxygen; SpO2 = oxygen peripheral saturation; ICU = intensive care unit; NS = not stated; RF = respiratory frequency; LU = lung ultrasound; SAH = systemic arterial hypertension; FiO2 = fraction of inspired oxygen; OTI = orotracheal intubation; CPAP = Continuous Positive Airway Pressure; HRCT= high resolution computerized tomography; ECMO = Extracorporeal Membrane Oxygenation; PaO2 = oxygen arterial pressure; ARDS= acute respiratory distress syndrome; % = percentage; USA=United States of America; SARS-CoV-2=Severe Acute Respiratory Syndrome Coronavirus 2.
Characteristics of the lung ultrasound and other image exams findings in COVID-19.
| Study | Lung ultrasound findings | Other image exams | Scanning areas/LU technique/sort of equipment | IPE and machine cleaning | Comments |
|---|---|---|---|---|---|
| Thomas et al.19 | Multifocal B lines; pleural and subpleural thickening; consolidation. | Thorax X-ray with bilateral infiltrates. | NS | NS | LU might be useful to manage COVID-19 suspected patients, even if it does not allow the differentiation of the viral pneumonia causes. |
| Soldati et al.12 | Small and large consolidated areas, pleural irregularities, blank vertical areas and extensive blank lung with or without consolidation. | None | LU score:0–3 points in 14 areas (three posterior, two lateral and two posterior in each hemithorax). Portable machines dedicated to exclusive use in patients with COVID-19. Convex or Linear probes, according to the patient’s body size. | Yes | Experience of the service in the standardization of COVID-19 assistance with emphasis on the need for a shared data base. |
| Buonsenso et al.15 | Irregular pleural line with small subpleural consolidations, blank lung areas, irregular and confluent vertical artifacts (B lines). Bilaterally present preserved areas and mixed with affected areas. | Thorax X-ray with doubtful left peri-hylar hypodiafania and HRCT (ground glass pattern). | A total of 12 areas were evaluated. Portable convex probe (3.5MHz). | Yes | Evaluation using portable LU and two examiners. |
| Kim et al.18 | B lines with variable aspect (focal, multifocal and confluent), subpleural consolidations, pleural thickening or irregularity and larger consolidations with occasional air bronchograms. | HRTC with ground glass opacity. POCUS for cardiac scan. | Handheld devices. Without probe description in the article. | Complete + ultrasound protective equipment. | Antisepsis care and preventing contamination during LU. |
| Denina et al.25 | Subpleural consolidations (2 patients). Confluent B lines (5 patients). Agreement with radiologic findings in 7 out of the 8 cases. One patient presented B line interstitial pattern, despite the normal thorax X-ray. | Thorax X-ray with consolidation in two patients and ground glass opacities. | Linear transducer from 7.5 to 13 MHz. | NS | LU showing high agreement with thorax X-ray examination; might reduce radiologic exams; able to stratify the patients according to severity into mild, moderate and severe; follow-up, exam repetition before discharge. |
| Yasukawa et al.26 | Glass rockets with or without Birolleau variant (white lung); confluent B lines and thick and irregular pleural lines; small subpleura consolidations (5 patients); large consolidation (1 patient). | X-ray and tomography. | NS | Yes | LU might be more sensitive than thorax X-ray in the diagnosis of interstitial syndrome. When resources are limited, where thorax radiograph, tomography and SARS-CoV-2 RT-PCR are not promptly available or the response time is long, LU might help COVID-19 diagnosis. |
| Musolino et al.27 | Vertical artifacts, white lung areas, subpleural consolidations and pleural irregularities. | X-ray (unspecified diffuse interstitial thickening), tomography (the findings of one case correlated to the LU findings) and resonance (signs of pneumonia). | Pocket wireless device. Sitting patients. Exam performed by 2 pediatricians with over 5 years of ultrasound experience. LU performed within 12 h of hospital admission. | Yes | Useful tool for the diagnosis and follow-up of COVID-19 related pneumonia. This study aimed at evaluating the LU role in COVID-19 child patients. |
| Ji et al.29 | Multiple B lines, small consolidations and pleural line thickening. | Thorax tomography (multiple bilateral and peripheral ground glass opacities). | NS | NS | LU showed reduction of B lines in the evolution (initially they were 88 and reduced to 18) with disappearance of consolidations. |
| Buosenso et al.30 | Irregularities in the pleural line. Consolidations with white lung area. Vertical artefacts. | Thorax X-ray was done in two patients only. Patient 1 was compatible with interstitial disease and patient 4 with hyperlucency and basal bilateral alteration. None submitted to tomography. | A total of 14 regions were evaluated. LU was carried out before the positive SARS-CoV-2 RT-PCR result. | NS | LU evaluation along the illness evolution showing improvement of the LU findings with relevant role in the therapeutic decision. |
| Inchingolo et al.31 | Diffuse hyperechoic vertical artifacts with thickened pleural liens and white lung with irregular distribution. | Thorax X-ray performed on the same day, did not suggest viral pneumonia. | A total of 14 regions (3 posterior, 2 lateral and 2 anterior in each hemithorax) along the paravertebral, middle axillar and hemiclavicular lines were evaluated. Convex wireless transducer (3.5MHz). | NS | LU was shown to be an accurate imaging method to detect pleural and peripheral pulmonary conditions, including pneumonia, with great accuracy, even in pregnant women. |
| Duclos et al.32 | A lines. Focal and confluent B lines. Pleural line thickening and irregularities. | Tomography: multilobar asymmetric lung lesions with peripheral distribution of ground glass opacities, consolidation, and crazy pavement pattern. | NS | NS | Direct comparison between LU and tomography and close time relation. |
| Zieleskiewicz et al.33 | Elderly, 72 years old: coalescent B lines and pleural line irregularities alternating with normal area. | Tomography: 72-year-old female patient: bilateral and multilobar ground-glass peripheral opacities. | NS | NS | Comparison between tomography and LU was carried out at the same time. Emphasizes the LU potential to evaluate COVID-19 associated pneumonia in several stages. |
| Elderly, 65 years old: pleural line irregularities associated with coalescent B lines, or multifocal subpleural consolidations. | Tomography: 65-year-old male patient: subpleural fibrosis, honeycomb, traction bronchiectasis with anterior distribution and interlobular septal thickening. | NS | NS | ||
| Youssef et al.34 | Pleural thickening. Bilateral diffuse coalescent B lines. | Normal obstetric ultrasound. | A total of 6 regions (2 anterior, 2 lateral and 2 posterior in each hemithorax) were evaluated. Linear or convex probes. | NS | Simplified LU systematic approach to motivate its adoption by obstetricians and gynaecologists. |
| Tung-Chen et al.,35 | Day 1 = A-lines; day 2 = pleural effusion; day 4 = subpleural consolidation; day 10 = diffuse B-lines; day 14 = irregular pleural lines and resolving B-lines; day 35 = A-lines. | Chest X-ray: local or bilateral patchy shadowing infiltrate. CT: ground glass opacities. | A total of 8 regions were evaluated. Curvilinear probe. | NS | LU guiding monitoring and therapeutic decision. |
| Lu et al.38 | 3 patients = normal aeration on LU; 27 patients = increased B-lines; 15 patients = coalescent B-lines (<3 mm); 5 patients = wide distance between B-lines (>7 mm) and the lung rocket sign; 3 patients = “white lung” sign; 6 patients = pulmonary consolidations including 2 with the presence of air bronchogram and 3 with shred signs; 3 patients = pleural thickening; 1 patient = pleural effusion; 1 patient = pneumothorax. | CT showed patchy ground glass opacities, consolidations, reticular shadows, small amount of pleural effusion. | Six regions (anterior superior, anterior inferior, lateral superior, lateral inferior, posterior superior and posterior inferior in each hemithorax) were evaluated and associated with a score method of 0–3 points in each area. Convex array transducer (2-5MHz) and linear array transducer (5-12MHz). | Yes | Comparison between LU score and tomography score was performed. Bedside ultrasound exhibits relatively low sensitivity with respect to lesions in the vicinity of the pulmonary hilum, which influences the quantitative assessment of lung lesions in patients with COVID-19. |
| Tan et al.62 | i. Thickened pleural line (12/12 patients); blurred or irregular (9/12 patients) and fragmented (6/12 patients); ii. Scattered B lines and comet tail signals (4/12 patients); partially diffuse (12/12 patients); completely diffuse with white lung (10/12 patients) or waterfall sign (4/12 patients); iii. Pulmonary consolidations or subpleural focal lesions <1 cm (5/12 patients); iv. Pleural effusion (1/12 patient). | CT with semi quantitative scoring method: ground-glass opacities, irregular pleural margin, septal or subpleural lines, honeycomb, subpleural cyst. | A total of 5 regions in each hemithorax were evaluated. BLUE protocol and BLUE Plus protocol were used in the study. The LU scoring system (i) pleural line involvement, including thickened, blurred, irregular or discontinuous pleural lines; (ii) lung parenchymal involvement, including B lines, partially diffused B lines, completely diffused B-lines (white lung) and lung consolidations; (iii) complications, including pneumothorax, emphysema, and pleural effusion. Portable device with convex array probe 2-5MHz. | NS | The study pointed out some differences in the LU findings in patients with COVID-19 related pneumonia and community-acquired pneumonia. The LU semiquantitative evaluation is viable to assess severity in interstitial pneumonia, including in patients with COVID-19. |
| Mafort et al.63 | B lines >2 (72.6%); coalescent B lines (36.2%); subpleural consolidations (8.06%). Unilateral lesions in 204 (49.9%) patients and bilateral in 205 (50.1%) patients. | Most patients were not submitted to CT. | A total of 12 areas (2 anterior, 2 lateral and 2 posterior in each hemithorax) were evaluated. Aeration score: 1 point=presence of >2 B lines; 2 points=presence of coalescent B lines; 3 points=presence of consolidations. | NS | Strong association between consolidation and dyspnea. LU findings can precede the patient clinical condition. LU shows prognostic capability in ARDS before evidence of hypoxemia, LU can define changes that affect the air/tissue relation on the lung surface. There is a correlation between LU and CT results with histopathological findings. In the study, the LU was not carried out in the patient follow-up/evolution and hospitalized patients were not included. |
| Veronese et al.64 | The most common findings of the study were not specified. | None. | A total of 12 areas (2 anterior, 2 lateral and 2 posterior in each hemithorax) were evaluated. LU score from 0 to 3 points, in which 1 point = presence of separated B lines occupying <50% of the pleural line; 2 points = presence of separated B lines occupying >50% of the pleural line; 3 points = lung thickening with a tissue aspect. | Complete | LU as mortality predictor = prognostic role. Greater accuracy for the LU when compared to the wrist oximeter. LU can be used in nursing homes or households. Study bias: small sample size and disregard of positive (such as medication therapy) or negative factors (such as comorbidities and polypharmacy). |
| Zieleskiewicz et al.65 | High diagnostic accuracy when compared to the X-ray in interstitial syndromes and alveolar consolidations. The most frequent LU findings were not specified in the study. | CT with ground glass opacity, consolidations and interlobular septal thickening. | A total of 12 areas were evaluated, with the posterior ones in the posterior axillary line, rather than accessing via paravertebral. | NS | LU score predictive of pneumonia severity, as evaluated in the CT and clinical characteristics. LU associated to severity evaluated by the CT and clinical parameters, with the possibility of substituting CT in the evaluation of the pulmonary involvement. POCUS for multiorgan evaluation: detection of deep venous thrombosis and acute right cardiac failure signals. |
| Yassa et al.66 | NS | Image with ionizing radiation in 1% — X-ray and CT (3 patients out of 296). | Convex transducer. A total of 14 areas (10 seconds per area) were evaluated. The patients were considered negative for SARS-CoV-2 infection suspicion when the LU score was 0 or 1 (in the absence of symptoms) point; however, patients were considered positive for SARS-CoV-2 infection in the presence of score 1 point and with symptoms, and scores 2 points and 3 points. | Complete | LU used to screen SARS-CoV-2 infection concluding that the LU findings were more sensitive than the maternal symptoms in the infection prediction. Potential to be used as a triage tool and in the evaluation of disease severity with the advantage of being used freely during pregnancy reducing exposure to radiation. |
| Zhao et al.67 | Most common findings in both groups were the B line patterns and shred sign. More ground glass opacity, consolidations and pleural effusion were observed in the refractory group. | Cites CT as gold standard, but does not explain its use in the patients of the study. | Convex transducer was used. A total of 10 areas (anterosuperior 2nd ICS and anteroinferior 5th ICS in the hemiclavicular line, laterosuperior 2nd ICS and lateroinferior 5th ICS in the mid-axillary line and posterior in the subscapular zone) were evaluated. LU score (from 0 to 40 points): 0 points = normal pattern with pleural sliding, parallel A lines and thin pleural line; 1 point = presence of B line patterns; 2 points = presence of ground glass signal with B lines occupying the whole screen; 3 points = presence of fragmentation signal similar to small subpleural consolidations; 4 points = presence of consolidation/pulmonary hepatization signal or pleural effusion. | Complete | All images were reviewed and scored by 2 medical doctors with over 5 years of experience in LU applied to critical care. LU used in the follow-up for evolution assessment: transformation of B lines into A lines; reduction and disappearance of consolidations; more consolidations and more interstitial syndrome pattern, which might mean disease worsening. LU can be used to evaluate aeration in critical patients. |
| Dargent et al.68 | B line patterns and consolidations. | CT: subpleural ground glass opacity with progressive extension and consolidations. | A total of 12 areas were evaluated. LU core from 0 to 3 points, in which 0 points = normal; 1 point =presence of well-defined B lines; 2 points = presence of coalescent B lines and/or subpleural thickening ≤15 mm and subpleural consolidations; 3 points = presence of consolidations (pleural thickening ≥15 mm), variation from 0 to 36 points. | NS | Higher score in deaths due to refractory hypoxemia and LU helped early diagnosis of pneumonia associated to mechanical ventilation. Good agrement between LU and CT for the presence of consolidations. |
| Bonadia et al.69 | Normal LU in three patients and pathological in 38 (92.7%) patients. | Pathological CXR in 34 (82.8%) patients and pathological CT in all patients submitted to examination (n = 17). | Portable, wireless device, with convex transducer. A total of 14 areas (2 anterior, 2 lateral and 3 posterior in each hemithorax) were evalauted during 10 seconds in each area. LU score from 0 to 3 points, where: 0 points = normal; 1 point = presence of regular or irregular pleural line with visible and non-confluent vertical artifacts; 2 points =presence of irregular pleural line with multiple and conludne vertical artifacts and/or subpleural consolidations; 3 points = presence of extensive and dense white lung areas with or without larger consolidations. | NS | Lung global assessment is mandatory, since each pulmonary area might be in distinct stages of the disease. LU carried out at the emergency room in the first evaluation is able to predict the global prognosis, the need for admission to the ICU and identify patients in greater death risk. |
| Deng et al.70 | Numerous and coalescent B lines with small multifocal consolidations in several regions — most common. | CT with ground glass opacity (96.1% patients), followed by consolidations (75.8% patients) and crazy paving pattern (ground glass opacity with overlapping of inter and intralobular septal thickening) (60.9% patients). | Convex transducer and adjusted around 10 cm deep. A total of 8 areas (2 anterior and 2 lateral in each hemithorax) were evaluated, in which the superior and inferior zones are delimited by the 3rd ICS. Images analyzed and scored by three blinded medical doctors with 3–6 years of experience. | NS | CT is not suitable for the follow-up of critically ill patients (despite being gold standard) due to transportation and medical team infection risks. |
| Presence of pleural line thickening and irregularities. | Most patients with bilateral and multifocal involvement. | LU score (0-24 points): 0 points = presence of A lines with pleural sliding or up to 2 isolated B lines; 1 point = presence of 3 or more spaced B lines restricted to a single ICS; 2 points=presence of multiple B lines (>50% of the area evaluated) with or without consolidations limited to the subpleural space; 3 points=presence of confluent or tissue-like pattern B lines, characterized by dynamic air bronchograms, defined as pulmonary consolidations. | LU carried out every 48 h after admission or when the examiners thought it was necessary. Change in score ≥2 points meant improvement, while ≤2 points meant worsening and = 1, unchanged condition. | ||
| Increase in B lines in different degrees and extension. | All patients showed peripheral pulmonary involvement. | The role of semi-quantitative scores in the follow-up of COVID-19 related pneumonia was studied. | |||
| Small and multifocal consolidations limited to the subpleural space. Also, consolidations in mass with dynamic air bronchograms ocurred. | Positive LU correlation with CT to evaluate the LU accuracy was higher in critical patients when compared to the severe ones. The patients had been diagnosed before the examination; therefore, the LU was used to evaluate the severity of the lesions, but not to diagnose the disease. The LU was more accurate in the evaluation of the worsening than the improvement or maintenance of the condition. | ||||
| Unusual pleural effusion and rare pneumothorax were found. | |||||
| Pagano et al.71 | LU used to verify alveolar recruitment after non-invasive CPAP. It was carried out before and after CPAP. | CT is considered gold standard in the quantitative evaluation of recruitment and pulmonary aeration, but it is not carried out due to certain issues such as transport logistics, contamination, instability of critical patients, among others. | A total of 12 areas (anterior, lateral and posterior, divided into upper and lower sections in each hemithorax) were evaluated. LU score from 0 to 3 points: 0 points = presence of A lines or below 3 isolated B lines; 1 point = presence of multiple and well-spaced B lines; 2 points = presence of coalescent B lines with or without small subpleural consolidations; 3 points = presence of pulmonary consolidation. Convex transducer. | NS | LU is a valid technique to assess alveolar recruitment, evaluation of extra-vascular pulmonary water and improvement after CPAP application. Patients that improved the PaO2/FiO2 relation after 1 h of CPAP showed lower mortality. |
| Martinez et al.72 | Pleural effusion in all patients, followed by diffuse and translobar subpleural consolidations, coalescent B lines and pleural line irregularities. No A line patterns were observed. Also, after a 3-month follow-up, all patients were asymptomatic, presented normal echocardiogram, no effusion and persistence of pleural thickening. | Cites CT as gold standard, but also the need for transfer of the critical/unstable patient, high infection risk and the ionizing radiation limits its use in children. | Linear transducer. A total of 10 areas (4 anterior — between the sternal and anterior axillary lines; 2 lateral — between the anterior and posterior axillary lines; 4 posterior — between the paravertebral and posterior axillary lines) were evaluated. | Cites sanitation/sterilization of transducers and the use of protection covers. | Infection in children is unusual and less severe. |
| Yu et al.73 | Case 1 = increased B lines and focal pulmonary consolidation. | Case 1 = thorax X-ray indicating pulmonary infection. CT with irregular shadows of high density and bilateral ground glass opacity. | Using remote ultrasound robotic device assisted by the 5G technology. | NS | Real time robotic scan using big data, cloud storage and artificial intelligence. A robotic arm provided the examiners with protection and reduced the number of professionals in contact with the patients with COVID-19. |
| Case 2 = partially thickened pleural line, intensive B lines, consistent with pneumonia signs. | Case 2 = multiple nodes with bilateral inflammatory appearance. | CT limitation in general application to all population groups (such as pregnant women and children). | |||
| Cho et al.74 | Early detection of B lines, in patients with normal X-ray, corresponding to the ground glass opacities in the CT. | CXR and CT at hospital admission, however, no findings were specified. | Microconvex transducer was used to evaluated 12 areas. BLUE protocol was applied and it was done the use of the Venice self-learning system with automatic identification of B lines (distinction from artifacts). LU score: 0 points = presence of ≤2 B lines; 1 point= presence of 3 or 4 B lines (B1 lines); 2 points= presence of ≥5 B lines (B2 lines); 3 points = presence of consolidation(s). | Not specified, but exemplifies complete garments | LU proved useful in the monitoring of the disease evolution. Also, LU in the triage of SARS-CoV-2 infected patients was able to indetify greater risk of respiratory failure. |
| Lichter et al.75 | Fragmented pleural thickening in 100 (83%) patients; irregular subpleural consolidations in at least one zone in 93 (78%) patients and pleural effusion in 9 (8%) patients. | CT with bilateral pulmonary infiltrates. CXR with bilateral infiltrates (39%); pleural effusion and rare lobar infiltrates (<15%). | Tranducer used for cardiac evaluation. A total of 12 areas (anterior, anterolateral and posterolateral in each hemithorax) were evaluated. LU score from 0 to 36 points: 0 points= presence of A lines; 1 point= presence of B1 lines (separated = moderate loss of pulmonary aeration); 2 points = presence of B2 lines (coalescent = severe loss of pulmonary aeration); 3 = presence of consolidation (complete loss of aeration). The pleural thickening was determined qualitatively. | Complete | LU identifies quickly the pulmonary involvement allowing stratification and prediction of the need for mechanical ventilation, mortality and outcome. The main factor responsible for the LU worse score was a new or greater involvement of the anterior pulmonary segments, a finding that can be used clinically as an alert of imminent clinical deterioration. |
| Lu et al.76 | NS | NS | Convex transducer (2-4MHz). A total of 12 areas were evalauted with LU score from 0 to 3 points for each of them (0–36 points in total). LU score: 0 (normal aeration) points= presence of pleural sliding with A lines or less than 2 vertical isolated B lines; 1 (moderate loss of pulmonary aeration) point= presence of spaced or coalescent B1 lines, multiple, well-defined or small juxtapleural consolidations; 2 ( severe loss of pulmonary aeration) points= presence of multiple vertical coalescent B2 lines or juxtapleural consolidations found in the whole area of one or two intercostal spaces and corresponding to alveolar edema; 3 points = presence of pulmonary consolidations with static or dynamic air bronchograms up to the complete loss of aeration. | NS | LU can be a more precise indicator of the ideal moment of intubation than the oxygenation index and the respiratory rate. |
| Dini et al.77 | Non-coalescent B lines in >3 zones (36 patients); coalescent B lines in >3 zones (32 patients); not consolidated hyperdense condition (30 patients); pleural effusion (11 patients). Pleural line abnormalities in 90% (irregularities, discontinuities and fragmentations). | None | Linear or convex portable wireless transducer. A total of 8 to 12 areas were evaluated. LU score from 0 to 3 points, where: 0 points=presence of normal pattern; A lines or insignificant B lines; 1 point=presence of non-coalescent B lines in >3 zones; 2 points=presence of coalescent B lines in >3 areas; 3 points=presence of not consolidated hyperdense condition. | Sanitation and disposable plastic packaging. Personal/Individual protection equipment not specified. | Serial evaluation strategy in population of older people in nursing homes and support institutions. |
| Iodice et al.78 | Multiple B lines and consolidations. White lung. | CT: bilateral multiple lesions; 80% showed bilateral ground glass opacity; 62% showed evidence of consolidation in the left lung and 69% had consolidation in the right lung; crazy paving pattern in 17%. | LU score were not informed. Convex (3-5MHz) and linear (9-12MHz) transducers. | NS | LU and CT carried out on the same day. Ground glass opacity showed correlation with the presence of B lines in LU and the crazy paving pattern correlated with white lung in LU. |
| convex (3.5-5 MHz) and linear (9- | |||||
| Tung Chen et al.79 | Bilateral, isolated or confluent B line pattern, pleural irregularity, presence of linear and subpleural consolidations. | CT in 51 patients: pleural thickening in 2 (1%); ground-glass opacity in 37 (72.5%); septal thickening in 18 (35.2%); crazy paving in 10 (19.6%); subpleural consolidation in 10 (19.6%); pleural effusion in 12 (23.5%). | A total of 12 areas with score from 0 to 3 points for each region evaluated (score from 0 to 36 points): 1 point= presence of irregular or isolated B lines; 2 points =presence of confluent B lines; 3 points = presence of consolidations or pleural effusion. | NS | Excellent correlation between CT and LU was observed. |
| Subpleural consolidations in posterior regions of the basal lobes were the most common finding. | CXR in 28 patients: ground-glass opacity in 12 (42.9%); interstitial pattern in 13 (46.4%). | Portable device and convex transducer (1.5–4.5 MHz). | LU showed accuracy similar to that of the CT to detect pulmonary abnormalities in patients with COVID-19. | ||
| Gregorio-Hernández et al.80 | Case 1 = LU in 3-day old patient without consolidation or coalescent B pattern; during evolution, presence of coalescent B lines and consolidation in the lateral and posterior areas.Case 2 = along the SARS-CoV-2 infection, more evident B pattern, mainly posterior with the appearance of consolidation. Blurred and thickened pleural line with normal pleural sliding.Case 3 = most areas with A pattern and thin pleural line with normal pleural sliding and isolated B lines; in posterior regions, thickened and blurred pleural line with coalescent B lines and millimetric pleural consolidation; during the evolution, the findings disappeared. | NS | Portable device with linear transducer. A total of 6 areas were evaluated. LU score from 0 to 3 points (score from 0-18 points): 0 points = presence of A pattern; 1 point=presence of ≥3 B lines; 2 points= presence of agglomerated and coalescent B lines; 3 points = presence of extensive consolidation. | NS | LU use in the follow-up, repeated 48/48 h in the first week after diagnosis. Apperance of consolidations and coalescent B lines did not follow the respiratory deterioration. |
| LeVine et al.81 | B lines | CXR: light bilateral irregular infiltrates. CT: ground glass opacities consistent with acute respiratory distress syndrome. | NS | NS | LU and other imaging methods allowed early diagnosis of COVID-19 despite its atypical clinical presentation. |
| Nouvenne et al.82 | Normal LU in 27 (33%) patients; bilateral multiple subpleural consolidations in 32 (39%) patients; diffuse bilateral B lines or white lung in 24 (30%) patients; focal B lines in 17 (20%) patients; pleural effusion in 3 (4%) patients; isolated abnormalities in the pleural line in 3 (4%) patients. | NS | Portable device with convex transducer (panoramic exploration) and linear (detailing the pleural line and subpleural alterations). A total of 8 areas were evaluated with LU score from 0 to 3 points (score 0–24 points): 0 points=presence of regular pleural line, presence of A lines; 1 point=presence of discontinued pleural line, focal B lines; 2 points=presence of fragmented pleural line, subpleural consolidations; 3 points=presence of white lung with or without consolidations. | NS | Bedside LU as auxiliary diagnosis in extra-hospital situations. Integration of anamnesis with clinic and LU allow the refinement of the diagnosis of respiratory diseases in the elderly, and might eliminate the need for avoidable hospital admission. |
| Yang et al.83 | 540 pulmonary regions were evaluated: multiple B lines in 324 regions; consolidations in 220 regions; pleural effusion in 67 regions. | CT showing 209 abnormal regions: ground glass opacities in 208 regions; consolidations in 16 regions; pleural effusion in 14 regions. | A total of 12 areas were evaluated.Considering regional alveolar-interstitial patterns such as multiple B lines (≥3) within the region evaluated using LU or the presence of ground glass opacities in the CT.Alveolar-interstitial syndrome was defined as the presence of ≥2 regions with alveolar-interstitial pattern per side and bilateral positivity. | NS | LU and CT carried out in an interval ≤12 h. LU was more sensitive tha CT in the diagnosis of regional alveolar-interstitial pattern, alveolar-interstitial syndrome, consolidation and pleural effusion. |
| Schmid et al.84 | Irregular pleural line with partially confluent B lines, mainly anterior and above the left lung. Presence of pleural sliding. Consolidation with hepatic echogenic texture, air bronchogram and pleural effusion in the right costophrenic sinus. | CT with ground glass opacities in the left apical lobe and consolidations in the right basal lobe. | NS | NS | Patient developed ARDS and multiple organ failure and died on the 14th day of evolution. |
| López Zúñiga et al.85 | LU use only in case 4: pleural line thickening and irregularity. Diffuse B lines and consolidations. | CXR in cases 1 and 2: unequal, diffuse alveolar-interstitial opacities, with peripheral predominance and pulmonary bases. CXR in case 4: no abnormalities found. CT in case 3: density diffusely increased with mainly ground glass peripheral bilateral distribution pattern, thickening of the interlobular sept or bronchiectasis. | NS | NS | LU proposed as an alternative for the diagnosis and monitoring of patients with COVID-19 with higher sensitivity than CXR, despite its low specificity. |
| Giacomelli et al.86 | Bilateral moderate B lines, without pleural effusion. | CXR with interstitial thickening in the right mid and basal field. | NS | NS | SARS-CoV-2 associated to increased risk of thromboembolism due to inflammation, stasis and hypercoagulability condition. Patient with no signs of distal hypoperfusion at admission and ultrasound examination confirming graft patency, the only possible explanation would be hypercoagulability and COVID-19 related inflammation. Patient died. |
| Nouvennea et al.87 | Bilateral involvement in 26 (100%) patients with predominance of basal, medial, or apical lobe involvement in 3 (12%) patients. Pattern of alveolar-interstitial syndrome: (i) with distinct B lines in 7 (27%) patients; (ii) with confluent B lines (white lung) in 17 (73%) patients; (iii) subpleural consolidations in 17 (73%) patients; (iv) parenchymal consolidations in 13 (50%) patients. LU score 15±5 points. | CT with, n (%): 26 (100) bilateral involvement, 21 (81) mixed axial distribution, 23 (88) involvement of 6 pulmonary lobes; 6 (23) predominance of basal, medial, or apical lobe involvement, 26 (100) Ground-glass opacities, 13 (50) subpleural lines, 15 (58) fat vessel sign, 4 (15) crazy paving sign, 2 (8) basal consolidations, 1 (4) centrolobular nodules, 1 (4) pleural effusion, 2 (8) lymphadenopathy. | Convex transducer for panoramic view and linear for abnormalities in the pleural line. A total of 8 areas were evaluated. LU score from 0 to 3 points, scores 0–24 (points): 0 points=regular pleural line and presence of A lines; 1 point=presence of fragmented pleural line, focal B lines; 2 points=presence of irregular pleura line, subpleural consolidations; 3 points=white lung with or without consolidations. | Exclusive transducers, machine and operator protection with IPE. | 34% deaths of patients in hospital treatment. LU score, according to type, extension and severity of the alterations, presented statiscally significant correlation with the CT severity score and SpO2 in ambient air. |
| Peyrony et al.88 | LU was used in 48 (21.4%) patients and bilateral B lines were identified in 36 (76.6%) patients. | CXR carried out in 80 (35.6%) patients. Findings consistent with normality in 19 (84%) patients. | Scores and evaluation technique are not mentioned, portable device. | NS | In COVID-19 suspected patients, anosmia, high clinical probability and presence of bilateral B lines in LU increased the probability of disease identification. |
| Rodriguez-Gonzalez et al.89 | Irregular pleural line, B-lines, some coalescent, with bilateral patchy distribution, and small peripheral consolidations, which were larger in posterior-basal areas. | Thoracic angioCT-scan ruled-out massive pulmonary thromboembolism but showed a pattern of ground glass and numerous consolidations of predominance in the posterior-basal segments of both lungs. | NS | NS | A concerning association between COVID-19 and the novel multisystem inflammatory syndrome has been recently noticed and increasingly reported. A severe cardiovascular involvement associated with pediatric COVID-19, even without previous heart disease. The screening of myocardial dysfunction and pulmonary. Hypertension through cardiac biomarkers or echocardiography could be beneficial in severe COVID-19 pediatric cases. Some SARS-CoV-2-infected patients who became critically ill suffered a generalized thrombotic microvascular injury mediated by intense complement activation involving the lung. |
COVID-19 = coronavirus disease 2019; NS = not stated; IPE = individual protection equipment; HRCT = high resolution computed tomography; CT = computed tomography; LU = lung ultrasound; CXR = chest-X ray; SpO2 = oxygen peripheral saturation; ARDS= acute respiratory distress syndrome; FiO2 = fraction of inspired oxygen; CPAP = Continuous Positive Airway Pressure; POCUS =point of care ultrasound; ICS = intercostal space; RT-PCR=real time polymerase chain reaction; SARS-CoV-2=Severe Acute Respiratory Syndrome Coronavirus 2; ICU=intensive care unit; PaO2=oxygen arterial pressure; BLUE=Bedside Lung Ultrasound in and Emergency.
Among the articles included, 17 are case reports,15,18,19,29,30,32,33–35,73,74,80,81,84–86,89 12 are observational studies,25,26,27,31,63,65,71,76–78,87,88 5 are observational prospective,66,68,69,75,79 and 4 are observational retrospective64,67,70,83 studies, 1 is a short communication,72 and 1 is a protocol.12
Detection of SARS-Cov2 on RT-PCR assay from the nasopharyngeal swab was found in 34 studies19,26,27,29,30,31,32,34,35,38,62–75,77–82,85–88 while the infection diagnosis was not specified in the other studies.12,15,18,25,33,76,83,84
The clinical picture was described in 39 studies and despite the variability in the signs and symptoms of the disease, fever15,18,25,26,27,29,30,31,32,34,38,62,63,69,70,74,75,77,78,79,82,84–88 was the most common symptom reported, followed by cough and dyspnea with 6 studies.15,19,21,25,26,28,29,32 It has been noted that in child populations, from 27 patients evaluated, aged 0–17 years old, 14 (51.8%) had fever, 11 (40.7%) had dry cough, and less commonly 3 (11.1%) had dyspnea, 3 had headache, 3 had odynophagia, 3 had vomiting and diarrhea, 3 had arthralgia and 2 (7.4%) had anosmia.27,31,72,74,80,89 Among the children evaluated in the studies, 4 were asymptomatic. In the adult and elderly population analyzed, the most common symptoms were fever, cough, and dyspnea, followed by headache, myalgia, nausea, vomiting and abdominal pain. The less common symptomatology included rhinitis, pharyngitis, anosmia and ageusia. Finally, within the sample of pregnant women totaling 17 patients, 6 of them had cough, 5 had fever, 2 had dyspnea, 1 had anosmia, 1 had chest pain and lastly for 11 of them, although symptomatic, their symptoms were not specified.30,31,34,66
The results of the studies revealed that patients presenting a clinical condition of dyspnea and hypoxemia showed alterations in the pulmonary aeration evaluated by LU. The evolution of the A line pattern into the appearance of B lines and consolidations was associated with the worsening of the disease and, consequently, of the clinical signals and symptoms.
The related comorbidities reported included hypertension, obesity, asthma and dyslipidemia, obstructive sleep apnea, rheumatoid arthritis, systemic lupus erythematosus, atrial fibrillation, end-stage kidney disease, dementia, diabetes mellitus, cancer and immunosuppression, liver disease, coronary disease, transient ischemic attack and stroke, and chronic obstructive pulmonary disease.
Regarding therapeutic measures, 19 studies did not mention the treatment employed. Some studies mentioned support measures, oxygen therapy and non-invasive ventilation19,25,26,29,35,67,69,70,74,76,78,80,87 or intubation with invasive mechanical ventilation19,33,36,67,69,70,76,80,81,86,89 and extracorporeal membrane oxygenation.67,70,74 The pharmacological interventions reported included the use of hydroxychloroquine, azithromycin, corticosteroids, oseltamivir, favipirapir, lopinavir/ritonavir, tolicizumab, sarilumab (anti-IL6), enoxaparin and broad-spectrum antibiotics,30,35,74,81,86,89 interferon inhalation therapy appeared in a single study,29 and another study reported the use of intravenous immunoglobulin.81
LU was implemented in the context of the SARS-CoV-2 infection in all studies and in 9 studies it was the only imaging test used in the propaedeutic of lung disease.12,34,64,67,71,72,77,80,82
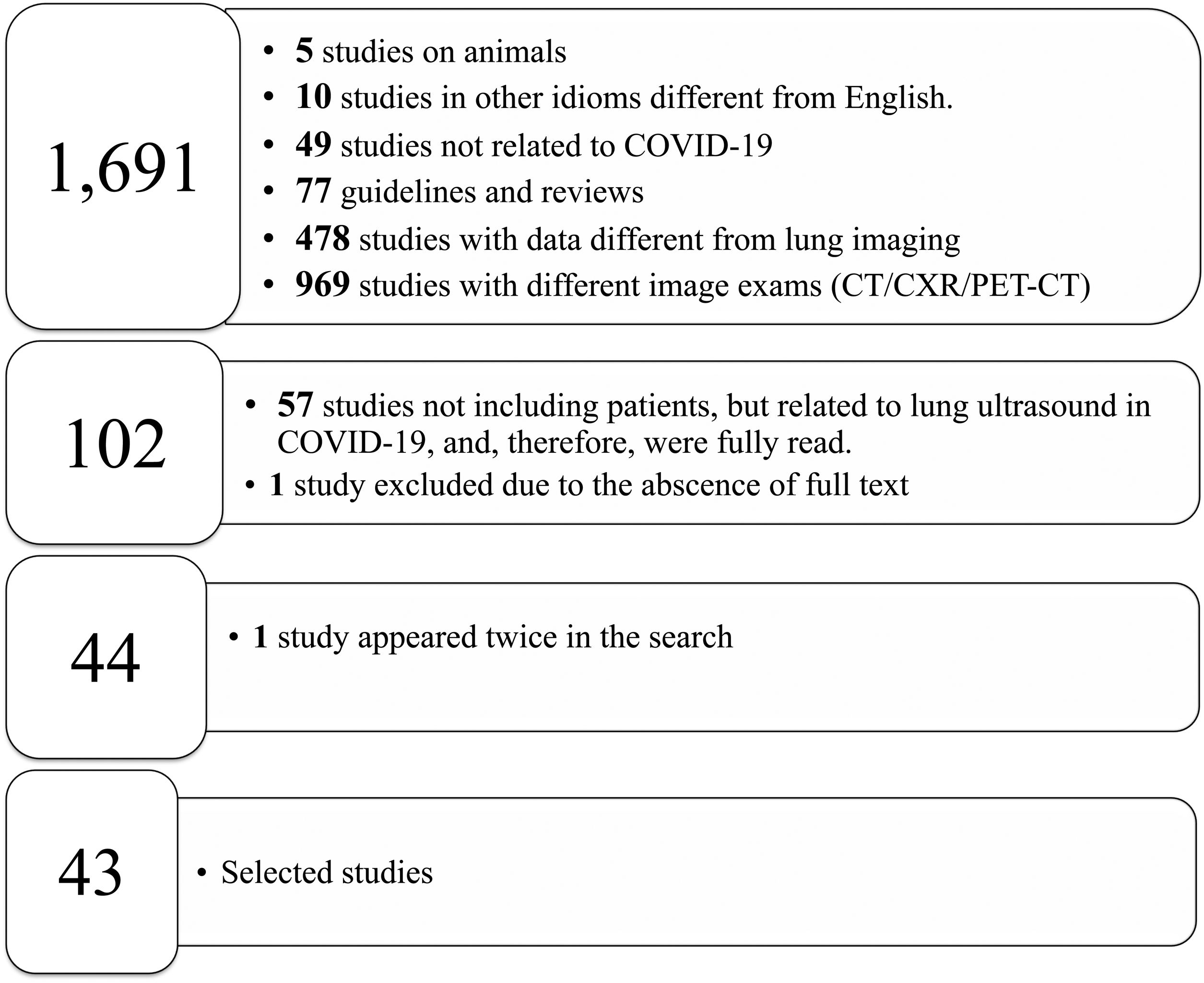
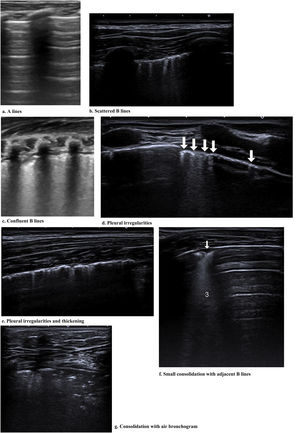
Most of the studies under analysis had LU findings in common in the presence of SARS-CoV-2 infection. The most common finding was B lines, which had very distinct characteristics, 34 of which reported focal, diffuse and confluent B lines.12,15,18,19,25,26,27,29,30,31,32–35,38,62,67,68,70,72–74,77–88 Twenty-nine studies described consolidations of different types: small, large, linear, subpleural, multifocal and translobar,12,15,18,19,25,26,27,29,30,33,35,38,62,63,67,68,70,72,73,75,78–80,82–85,87,89 associated with white lung12,30 and with air bronchograms18,38. Nineteen studies described pleural irregularities,12,15,18,26,27,30,32,33,35,62,70,72,77,79,80,82,84,85,89 and 19 reported pleural and subpleural line alterations, such as thickening or shred sign.18,19,26,29,31,32,34,38,62,67,70,72,73,75,77,80,85 Ten studies referred to pleural effusion,38,62,67,70,72,75,77,82–84 8 reported bilateral findings15,34,63,79,82,86,87,88 and 3 revealed preponderance of compromise in posterior areas.79,80,89 Three studies associated appearance of A lines during recovery 35,67,80 and most of the papers reported the LU role in the serial evaluation of patients with COVID-19.27,29,30,33,35,67,68,70,71,72,74,75–77,80
Other characteristics found were the presence of glass rockets38 with or without the Birolleau variant,26 also known as white lung.61
Some studies reported radiological findings agreement between LU and HCRT.15,18,25,27,29,32,33,35,38,62,63,65,68,70,78,79,83,84,87,89 The main HCRT findings were ground glass opacities, “crazy-paving” pattern, consolidations, pulmonary infiltrates and pleural thickening as well as interlobular septal thickening.18,29,32,33,35,38,62,65,68,70,73,75,78,79,81,83–85,87,89 When comparing thorax HRCT features with those of LU in pneumonia resulting from COVID-19 the findings included (i) correlation of ground glass opacity with B lines (multifocal, discrete or confluent); (ii) presence of thickened pleura in HRCT with thickening of the pleural line in LU; (iii) pulmonary infiltrates as confluent B lines in LU; (iv) pulmonary consolidation in both techniques20 The finding “white lung” was also described as ground glass opacity in HRCT.15,18,26,27,38,62,78,87
The areas evaluated during LU presented varied results between studies; with the evaluation from 4 to 7 regions in each hemithorax depending on individual studies,12,15,30,31,34,35,62–72,74–77,79,80,82,83,87 totaling 8, 10, 12 and 14 assessment areas in these papers. Differences were found in relation to the type of transducer used, whose choice was related to the propaedeutic strategy of better evaluation of superficial regions or smaller thoraces such as in children, using the linear probe, or deeper regions and larger thoraces using the curved probe.12,15,25,31,34,35,38,62,66,67,69–72,74,76,77–80,82,87 Only one study employed sectorial probe in the pulmonary evaluation, since the institution protocols of that study advocated the point-of-care cardiac evaluation.75
Eighteen studies used LU scores.12,38,62–64,67–71,74–77,79,80,82,87 One study proposed a unified approach to standardize the use of LU in the clinical management of patients with COVID-19,12 with a score system to classify the seriousness of the lung disease (score): (0 points) presence of A lines with continuous and regular pleural line; (1 point) presence of visible B lines and irregularities in the pleural line; (2 points) presence of discontinuous pleural line with dark areas under the pleura (consolidations) with associations of B lines; (3 points) presence of coalescent B lines with “white lung” aspect, dense and widely distributed, with or without consolidations. In that study, 14 thoracic regions were evaluated, using a LU score from 0 to 42 points, in which higher score represented higher severity. Another study38 applied an LU scoring method ranging from 0 to 36 points in which both hemithoraces were divided into six regions (totaling 12 areas) where: (0) points = presence of pleural line and A-line, <3 B-lines; (1) point = presence of more than 3 B-lines; (2) points = presence of coalescent B-lines; (3) points = presence of pulmonary consolidation signs. The same assay presented a classification of severity of lung lesions: none (0 points); mild (1–7 points); moderate (8–18 points), severe (≥19 points).40 A third assay showed a scoring system of three items: (a) pleural line involvement, where (0) points = normal; (1) point = thickening (≥0.5 mm) or irregular; (2) points = blurred; (3) points = discontinuous, fragmented; (b) lung parenchymal involvement, where (0) points = no B-line; (1) point = presence of B-line ≤3; (2) points = presence of B-lines ≥4 or partially merged; (3) points = presence of B-line fully integrated (white lung or waterfall sign); (4) points = presence of pulmonary consolidation or subpleural lesion; (c) complications, where (0) points = none; (4) points = am line (pulmonary balloon); (4) points = pneumothorax or empyema; (4) points = pleural effusion. Pleural line, pulmonary parenchyma and complications were observed and scored respectively in each of the 10 examined sections.62 Also, Zhao et al. proposed a scoring system from 0 to 4 points imputed to 10 lung sonographic areas, as described below: (0) points = presence of normal pattern with lung sliding, parallel A-lines and thin pleural line; (1) point =presence of B-lines pattern; (2) points = presence of ground glass sign with B-lines occupying the entire screen; (3) points = presence of shred sign suchlike small subpleural consolidations; (4) points = presence of consolidation/pulmonary tissue-like aspect or pleural effusion.67
Other particularities and LU scores found in the studies listed above are detailed in Table 2.
The description of individual protection equipment use during LU examinations was carried out in 14 studies only.12,15,18,26,27,38,64,66,67,72,74,75,77,87 The common strategy to minimize the risk of transmission was making a LU machine available exclusively for COVID-19 exams combined with the use of protective covers for the probe and equipment. The LU examination had to be carried out, if possible, by 2 professionals, one of whom would be in direct contact with the patient and the other with the screen, the keyboard and the image acquisition and recording.12,27,67 Another strategy employed to reduce the occupational risk when dealing with patients with COVID-19 was the use of portable wireless transducers, making it easier to clean and handle the equipment.12,27,31,62,69,73,77,79,80,82,88
Fig. 2 shows the main findings of the LU imaging described in the study.
DiscussionIn the COVID-19 pandemic, it is necessary to seek tools that enable the evaluation of lung impairment by the disease, minimizing the involvement of multiple teams and the exposure of professionals in the health area to the SARS-CoV-2. The LU examination is an alternative in the respiratory system propaedeutic as it is a low-cost technique, highly portable and allows for repetition of exams, and can be performed at the patient’s bedside.12–14,20,22,55 The LU may be used in several moments of the natural history of the SARS-CoV-2 colonization/infection as it can identify the pulmonary involvement and seriousness of the disease in patients with suspected or confirmed COVID-19. It can also help to reduce the use of X-ray and/or thorax HRCT.17,62,66,74,85 In addition, LU is a low-cost tool that can be used in low- and average-income countries where HRCT might not be available,12 however, specialization in the area is necessary to use this technique.
The application of LU in the screening of COVID-19 symptomatic patients in the pre-hospital phase through pneumonia evaluation was described.13,63,64,82 Dini et al. (2020) proposed a flowchart of the intervention carried out employing LU in the evaluation and triage of older individuals living in nursing homes that presented symptoms consistent with the SARS-CoV-2 infection and those exposed to the infection from having contact with patients with COVID-19. Those exposed to patients with COVID-19 were submitted to LU and when the result was negative, pneumonia was excluded. When the LU result was altered, nasopharyngeal material was collected for RT-PCR. Whenever the result was positive, isolation and treatment were started; in addition, LU was carried out every 5–7 days and if the symptoms worsened, hospital admission was indicated. Patients that presented clinical symptoms consistent with COVID-19 and whose LU showed altered results, were isolated, treated and their pulmonary condition was monitored with ultrasound. Those showing unaltered LU were subject to SARS-CoV-2 RT-PCR collection and when the result was negative, other etiologies were considered for a differential diagnosis.
Other studies also showed LU applicability in the home assistance of older people and helping prevent unnecessary hospital admission, since LU integrated to the clinic and physical examination resulted in more accurate diagnosis of COVID-19 and other respiratory disease in older populations. In addition, due to the overload of the health service caused by the pandemic, some countries implemented home care for the older population whenever possible.64,82
Additionally, LU can be used in suspected diagnosis and in the prognostic stratification of individuals with pneumonia through the extension of specific patterns and their evolution to the consolidation phase in emergency assistance; it makes possible the management of patients in intensive care in relation to the mechanical ventilation and ventilator weaning; it can also monitor the effect of the therapeutic measures, like alveolar recruitment maneuvers, implemented in seriously affected patients submitted to invasive mechanical ventilation with orotracheal intubation.30,71,75
In clinical practice, when managing patients with acute hypoxemic respiratory failure due to COVID-19, deciding whether to proceed with the invasive mechanical ventilation and intubation might be a challenge; LU might be a helpful and accurate indicator of the ideal moment for intubation.76
Lu et al. investigated the role of LU role in the evaluation of the severity of the pulmonary aeration loss in intubated patients due to pneumonia by SARS-CoV-2 and those that were not intubated. The study carried out LU evaluations at different moments in the hospital care, within the period of one week after the patients had been admitted in the intensive care unit. The author pointed out that the LU could evaluate dynamically the ventilation condition of the two groups of patients in the study during the treatment and enable the prediction of the disease decline.76
Since a LU negative result, that is, without visible alterations, does not rule out a SARS-CoV-2 infection, LU cannot be considered a tool that substitutes the physical examination or the SARS-CoV-2 RT-PCR, but, it should be considered a complementary tool to be used in the screening of patients to detect mild symptoms and allow a fast and efficient decision.82 However, it has become clear that in the COVID-19 pandemic, the characteristics suggesting alterations provoked by the disease appearing in a LU or in the HRCT, even in the event of a negative SARS-CoV-2 RT-PCR test, might be highly suggestive of a SARS-CoV-2 infection.35
Antúnez-Montes et al. emphasized LU usefulness in the evaluation and triage of patients that presented respiratory complaints in the context of the SARS-CoV-2 pandemic.41 Abnormal LU findings would lead to a possible early admission into emergency units or intensive care units, while the identification of normal ultrasound patterns would categorize those patients as low risk patients. Fox et al. pointed out that LU presented a potential role in the triage of patients infected with the SARS-COV-2 and suggested an association between the more noticeable LU alterations and clinical deterioration.42,63,66,69
The existing literature suggests using of HRCT for the COVID-19 diagnosis and as a triage tool to identify SARS-CoV-2 infection, because, although the nasopharynx swab presents a definite etiological diagnosis, this test also presents limitations, mainly due to its low sensitivity,27,43 which is lower than HRCT.44 However, the HRCT is represents high cost, low availability, exposure to ionizing radiation that limits its use in some populations such as pregnant women, and the need for sedation in lower age groups.16,27,66 Therefore, LU becomes an important tool for the triage and evaluation of patients presenting COVID-19 symptoms.45,66,74
Pulmonary abnormalities might occur before clinical manifestations and some specialists recommend HRCT for patients with clinical suspicion of COVID-19.20,47 However, the high contagiousness of this virus and the risks of transporting patients in hemodynamically unstable and invasive mechanical ventilation to where radiography can be performed, results in the need for alternatives to evaluate lung damage. In such a context, LU is a positive choice as it provides similar results to those of the HRCT and is a more advanced method than thorax radiography for the evaluation of pneumonia and/or respiratory distress syndrome in adults, with the advantage of being an ionizing radiation free method.22,26,31 The LU findings in pneumonia and respiratory distress syndrome in adults caused by the SARS-CoV-2 are related to the illness phase, the seriousness of the pulmonary lesion and the presence of comorbidities. The predominant pattern is of varied degrees of interstitial syndrome and alveolar consolidations, related to the seriousness of the pulmonary lesion.20,26,30,82,83
Earlier quantification of the severity of the pulmonary impairment in patients with COVID-19 might be obtained by estimating the total number of pulmonary areas detected as pathological in LU.48,83 In a prospective study with 10 patients during a 15-day investigation period, the author demonstrated the LU score as a bedside non-invasive monitor of the pneumonia evolution in the COVID-19 disease by the graphical description of the evolution of the scores in the LU. Successfully extubated patients showed lower scores than those found before the intubation. The scores tended to increase in deaths caused by refractory hypoxemia, as a result of the progressive component of the pulmonary aeration failure characteristic of COVID-19. In addition, the score enabled early diagnosis of pneumonia associated with mechanical ventilation through the increase in new consolidations. Good agreement was observed between the thorax HRCT and LU to detect the presence of consolidations.68
In an observational series, LU was shown to be a useful tool to evaluate and monitor lung impairment in pregnant women with COVID-19, playing an important role in the treatment decision. All the patients presented ultrasound characteristics indicating COVID-19 at admission and 3 patients obtained resolution of the ultrasound pulmonary alterations.30 The use of LU in the follow-up and evolution evaluation has been described in several studies.67,70,80 Zhao et al. evaluated 35 patients with different clinical conditions and divided them into two groups that were classified according to clinical severity. Patients feeling uncomfortable and with respiratory failure were called non-refractory, while those presenting refractory respiratory failure with PaO2 (oxygen partial pressure)/FiO2 (oxygen inspired fraction) ≤100 or patients treated with extracorporeal membrane oxygenation, were classified as refractory group. The ground-glass opacity pattern and the consolidations were more frequent in the refractory group. In the follow-up, B lines were seen to transform into A lines, with reduction in and disappearance of consolidations. Therefore, it seems relevant to emphasize that the presence of more consolidation areas and interstitial syndrome might imply disease worsening. In addition, the study showed one more use for the LU to spot, with high specificity (∼90%), patients that might need extracorporeal membrane oxygenation.
When evaluating the child population, a study with 8 individuals from 0 to 17 years old documented improvement of radiological alterations throughout clinical evolution and resolution of the pulmonary disease caused by the COVID-19. One of the patients that presented a severe clinical situation was examined repeatedly with LU on alternate days and a reduction was noticed in the B lines pattern bilaterally one day before the clinical and radiographic improvement. In the same study, LU repeated before the hospital discharge showed improvement in the resolution of consolidations and interstitial patterns, consistent with the concomitant radiologic findings.25 The reappearance of A lines was reported in the illness recovery phase.35,56,80
Gregorio-Hernández et al., described LU findings in newborns infected by SARS-CoV-2, carried out every 48 h in the first week after diagnosis, and ascribed a score according to the severity of the pulmonary sound involvement and applied the score (from 0 to 3 points) to the 6 anatomical regions under evaluation. Newborns with an initial score from 3 to 4 points presented some degree of interstitial alveolar syndrome, and the newborn with the highest score evolved with the worst respiratory outcome.
Dudea et al. proposed a severity classification through the evaluation of the pulmonary damage using LU, in which mild to moderate damage was seen as B lines in growing number and distribution, irregular pleural line with interruptions, heterogeneous mixture of B and A lines A and pleural areas with normal sliding and small subpleural consolidations (<1 cm); the severe damage involved several B lines, stretching in superior and anterior directions, with confluent areas and increase in the number and size of the subpleural consolidations, and critical damage presenting coalescent B lines, extensive damage to the anterior and superior pulmonary areas, numerous small consolidations, areas of bilateral alveolar filling syndrome with or without air bronchogram and rare pleural collections.46 The topographic analysis of lung regions by LU and the application of scores seem to be efficient strategies for patients that require intensive care. LU scores are important to evaluate the seriousness of the structural impairment and enable the establishment of correlations with clinical deterioration.12,17,38,67,69,70 A step-by-step approach to safely performing LUS was described in several studies and recommended a systematically scanning of different zones in both hemithoraces proposing a point-awarding system to the LU findings and their correlation to the severity of the lung impairment.17,49,69 Several studies reported scanning of three areas of the thorax: anterior, lateral and posterior segments and, next, superior and inferior. Thus, 6 regions were defined for each lung.15,17,34,38,63–65,68,71,74–76,79,83 Points were awarded according to the region and the distinct aeration patterns [point(s)]: [A lines] = 0 points; [B1 lines — are associated with an interstitial syndrome and diminished lung aeration] = 1 point; [B2 lines — confluent lines appearing as a “white lung” (called also glass-rockets), equivalent to computed tomography (CT) ground-glass opacities] = 2 points; [consolidations] = 3 points. Therefore, the LU score ranged from 0 (normal) to 36 (worst pulmonary aeration).17
A retrospective study by Yan et al. analyzed 540 pulmonary regions of 29 patients with COVID-19 related pneumonia confirmed in China. The patients were submitted to simultaneous LU and HRCT in intervals of time under 12 h. That author defined two patterns of how the disease affected the pulmonary system: the interstitial alveolar pattern, defined by multiple B lines (≥3) within a region found in the LU or the presence of ground-glass opacity in the HRCT; and the Interstitial Alveolar Syndrome, defined by two or more regions with interstitial alveolar pattern per side, in which both should be found bilaterally. LU was seen to be highly sensitive to alterations in the air/pulmonary liquid interface, showing clearly the interstitial alveolar damage with inflammatory exudates and edema caused by the COVID-19. Therefore, LU showed higher sensitiveness than the thorax CT in the identification of alveolar and interstitial disorders, consolidations and pleural effusion.83
Another study adopted a new protocol called CLUE (COVID-19 Lung Ultrasound in Emergency) involving an anatomical parameter,49 a LU scoring system and oxygen requirement at the time of examination, in order to help the emergency clinician to make therapeutic decisions. The LU scoring system was reported as a valid tool for assessing regional and global lung aeration in Acute Respiratory Distress Syndrome in other studies,50,51 one that can be used in COVID-19 pneumonitis with several similar sonographic features.20 LU scoring system points ranged from 0 to 3 points, in each zone, totaling 12 zones, with higher points allocated to severe lung changes. The severity was classified as mild (score 1–5), moderate (6–15) and severe (>15). Employing the CLUE protocol could help risk-stratify suspected patients with COVID-19 and may decrease reliance on chest X-rays or HRCT chest during the initial clinical evaluation of suspected patients.
Two studies have evidenced LU accuracy in detecting acute illnesses and its non-inferiority in relation to thorax radiography in the differential diagnosis of pneumonias, pulmonary edema and pleural effusion;15,23 the use of this tool in chronic diseases such as cystic fibrosis was also evaluated.24 This demonstrates the advancement of ultrasound technology for pulmonary evaluation in the last few years and the relevance of this tool being disseminated in the management of COVID-19, not only for risk groups (elderly, pregnant women and adults with comorbidities) but also in the pediatric community.
When evaluating the findings of interstitial pneumonia in COVID-19, it is possible to obtain images that help the cardiovascular semiologic evaluation by providing information on the presence of morbidities such as cardiopathy and becoming a guide to fluid therapy and hemodynamic stability. Based on Point of Care Ultrasound (POCUS) findings in several organs, there are reports in which the patients received intravenous bolus injection of saline solution for hemodynamic resuscitation. The cardiac and inferior vena cava evaluations are easily obtained, and this approach aids determination of other dyspnea causes. Therefore, ultrasound might guide the amount of intravenous fluid to be used and also evaluate the right ventricular function when there is suspected cardiac insufficiency, in cases where COVID-19 might be co-occurring with cardiovascular comorbidities.57,65,72,75
POCUS can be used to quantify the illness course by counting the number of B lines in different lung regions, consolidation size or pleural effusion and development or resolution of any other lung abnormalities.53 Ji et al. used the B line count as a semi-quantitative index representing series of alterations in the pulmonary lesions before and after treatment,29,58,62 however, with some reservation, since B lines might be found in other cardiac and pleuropulmonary diseases.26,52 Other POCUS applications listed were the evaluation of deep venous thrombosis, assessment of endotracheal tube, mechanical ventilation weaning with evaluation of edema in the airways and diaphragm mobility, tracheostomy and sleep-guided deep vascular access.53,54,57,71,89
Despite the LU nonspecific findings that can occur in other illnesses including other viral pneumonias and a broad spectrum of non-infectious diseases (chronic obstructive pulmonary disease, neoplastic lymphangitis, pulmonary fibrosis and interstitial pulmonary disease), the homogeneous interstitial pattern seems to suggest the cardiogenic edema diagnosis, while a heterogeneous interstitial pattern, mainly in combination with subpleural consolidation and/or pleural thickening might suggest, in the pandemic clinical and epidemiological environment, pneumonia caused by the SARS-CoV-2.59 One study investigated ultrasound particularities that could help the distinction of bacterial pneumonia and the pneumonia caused by SARS-CoV-2. The differentiation by LU between viral and bacterial pneumonia is a challenge, however, in that assay the finding of B line artifacts, or partial or confluent B lines, small consolidations and pleural alterations was suggestive of the COVID-19. These findings are described in other interstitial pneumonic processes of viral etiology.62 Isolated large lobar consolidation with or without pleural effusion and with dynamic air bronchograms indicates bacterial infection.60
Some studies used the contrast-enhanced ultrasound to evaluate lungs regarding the presence of an underlying thrombosis process. Bedside contrast-enhanced ultrasound was indicated due to the presence of small and cuneiform pulmonary consolidations with a central echogenic point (signal described in pulmonary embolism patients) associated to the discovery of high levels of d-dimer and the impossibility of performing contrast-enhanced HRCT. Large delimited perfusion defects were demonstrated in relation to the normally perfused parenchyma. These areas were solved with the clinical improvement.37,39,46
LimitationsThe point of the disease development at which the ultrasound evaluation is performed is extremely important since the structural damage might precede clinical alterations.
The observation studies were seen to present small samples and experimental clinical studies were scarce.
Besides the high number of studies, the use of LU as a triage tool still lacks specific protocols, operator experience as well as reproducibility and inter-operator agreement.42,45,46 Further studies are needed to determine the role of LU as a triage tool and its use in the prognostic and monitoring of hospital patients.47
Due to the model of register of papers on the PubMed, some studies that might possibly include data collected from patients might have been overlooked in the filter analysis.
HighlightsLU findings presented correlation with HRCT images.
LU can be used in respiratory system propaedeutics as an alternative to the “stethoscope use”. Special clothing and individual protection equipment are indispensable, since the manipulation of the stethoscope in pulmonary evaluation might create contamination risks for the health professionals and patients.
COVID-19 normally induces a bilateral and diffuse interstitial pneumonia with asymmetric lesions and uneven distribution, mainly involving the lung periphery, which makes it particularly suitable for investigation using LU.60
Studies have identified potential correlation between the LU patterns and the patients’ clinical outcome. One of the assays in this study reported that each pulmonary area could be in a different stage of the disease, therefore, the global evaluation of the lungs is fundamental.69
The POCUS allows for hemodynamic, cardiac and vascular evaluations (thromboembolic phenomena — deep venous thrombosis).
LU should be associated to the multisystem point-of-care exam, since the SARS-CoV-2 infection might be linked to myocarditis and a high incidence of thromboembolic events. Thus, multiorgan ultrasound evaluation in early treatment is useful to screening these complications at the bedside.
PerspectivesMore studies on LU application in the pediatric population are necessary.
LU in COVID-19 score standardization.
Improvement of reading/automatic identification of B line software, as reported in this study is still needed.74
The advancement of the remote robotic ultrasound scanning technology assisted by the 5G network in real time by the use of big data, cloud storage and artificial intelligence must be improved.73
ConclusionsLU can be employed in different age groups to evaluate the seriousness and the response to treatment for the COVID-19 control. The main characteristics of LU in COVID-19 are focal, multifocal and/or confluent B lines, corresponding to the ground glass opacity of the thorax HRCT, in addition to the evidence of pleural thickening and irregularities. The fact that findings correspond shows the potential of LU as a radiation free, low cost, safe, reproducible method, with easy-to-sanitize equipment. Another advantage is the reduced need to manipulate the patient compared to the HRCT, avoiding transportation of the patient to the X-ray room and reducing the risk of contamination of other patients and the health professionals directly or indirectly involved with the patient. With the increase in the number of studies on the application of ultrasound, LU has been shown to be a useful tool for evaluating the progression, therapeutic response and follow-up of pulmonary disease in COVID-19.
Conflict of interestThe authors have no conflicts of interest to declare.
Financial supportThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributions[AOP, RMC, RU and FALM] participated in data collection; [AMAF, JDR and FALM] supervised, performed and validated data based on reproducibility. All authors conducted the writing and critical review of this study. Also, all authors read and approved the latest version of the manuscript prior to submission.
The authors are grateful to the professionals in the teams of the referenced pediatric urgency and emergency and pediatric pulmonology units as well as to the University of Campinas radiology department. Also, the authors thank Fernando Belluomini for collaboration in the data collection.
The following are Supplementary data to this article:
Lung ultrasound in COVID-19: a systematic review.