There is evidence of short- and long-term impairment of physical performance in patients with COVID-19 infection, but a verification of measures of physical impairment in this condition is lacking. We reviewed the measures used to assess physical performance in these patients. Secondary targets were measures of exercise or daily life activities induced symptoms.
MethodsMedline, CINAHL, and Pedro databases were searched from January 2020 to February 2021 for articles in the English language. Two investigators independently conducted the search, screened all titles and/or abstracts based on the inclusion criteria and independently scored the studies. The quality of the studies was evaluated by two reviewers according to the NIH quality assessment tool for observational cohort and cross-sectional studies. Discrepancies were resolved through consensus.
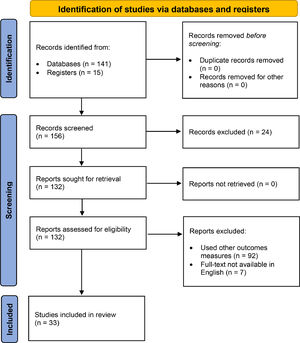
ResultsOut of 156 potentially relevant articles, 31 observational studies (8 cross-sectional), 1 randomized controlled trial, and 1 protocol were included. The quality of most of the 31 evaluable studies was judged as low (11 studies) or fair (14 studies). Sample sizes of the studies ranged from 14 to 20,889 patients. among the 28 reported measures, Barthel Index (42.4% of studies), Six-Minute Walking Distance Test (36.4%), Short Physical Performance Battery (21.2%) and 1-Minute Sit-to-Stand (12.1%) were the most used. Fifteen% and 36% of studies reported exercise induced desaturation and dyspnoea when performing the assessments, respectively. Other exercise induced symptoms were fatigue and pain. Studies reported wide ranges of impairment in physical performance as compared to “reference” values (range of mean or median reported values vs “reference values”: 11–77 vs 100 points for Barthel Index; 11–22 vs 22–37 repetitions/min for 1m-STS; 0.5–7.9 vs 11.4 ± 1.3 points for SPPB; and 45–223 vs 380–782 m for 6MWT respectively).
ConclusionThis review found that a wide variety of functional status tests have been used, making comparisons difficult between studies. These measures show impairment in physical performance in COVID-19 patients. However, the quality of most of the studies was judged as low or fair.
Clinical presentation of COVID-19 varies widely, ranging from no symptoms or light flu to pneumonia with acute respiratory failure requiring admission to the Intensive Care Unit (ICU) and possible death.1–3 In addition to the physiological consequences, a high prevalence of impairment in physical performance is reported in patients recovering from COVID-19.4–7 In patients without previous disabilities, maximal voluntary contraction for quadriceps and biceps was found to be 54% and 69% of predicted values, respectively.4 In another study, 76% of patients reported at least one symptom, and 23% reported anxiety or depression up to 6 months after acute infection. The most common symptoms were fatigue, muscle weakness, or sleep difficulties.5
Thus the need for validated measures is of utmost importance, using safe equipment and procedures,8 to evaluate the short- and long-term consequences of COVID-19. To the best of our knowledge, a review of the measures of physical performance used during the pandemic in COVID-19 patients is lacking. Standardisation of batteries of measures would allow us to make comparisons to be made among studies and the different follow-up time-points.
Therefore, we reviewed the measures used to assess physical performance in these patients. Secondary targets of our research were the measures of exercise or activities of daily life (ADL) induced symptoms.
MethodsWe performed a mapping review, defined as a systematic search of data in a broad research field of the knowledge, and their presentation as a visual synthesis (map).9 This study followed all Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and reported the required information accordingly.10
Search strategyMedline, CINAHL, and Pedro databases were searched from January 2020 to February 2021 for articles in the English language. We also searched the references of retrieved articles to identify possible additional studies. Keywords used were COVID AND “physical performance” OR “functional status” OR “disability” OR “impairment” OR "physical function" OR “activities of daily life” OR "muscle function" OR “exercise tolerance” OR “exercise capacity” OR “exercise-induced desaturation” OR “dyspnoea” OR “rehabilitation”.
Inclusion criteria: The search was limited to randomised controlled trials (RCTs), observational (including cross-sectional) studies, and protocols, which used at least one measure of physical performance, either patientreported by means of questionnaires, or objectively measured by means of standardised test such as exercise, functional performance or functional capacity. For the purposes of this review, a measure was defined as quantitative data described in the study. As secondary targets we searched also the measures of exercise- or ADL-induced symptoms.
We included all studies on COVID-19 patients, diagnosed either by positive test using a swab from upper or lower respiratory airways or by clinical or radiological findings. No restrictions were placed on the procedures used to diagnose COVID-19 or on the setting (hospitalization, rehabilitation, follow-up). No restriction was applied regarding age, ethnicity or sex.
Exclusion criteria: Studies not reporting any measure of physical performance (e.g. studies measuring only lung function, blood chemistry, etc.), were excluded. Systematic reviews, case report and case series were also excluded. In terms of the quantitative description of measures, we excluded studies with data reported as other than mean (standard deviation: SD) or median [Interquartile range: IQR].
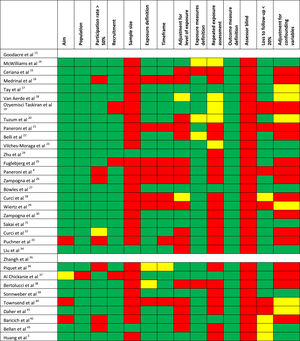
Quality assessmentThe methodological quality of the studies was evaluated using the National Institute of Health (NIH) quality assessment tool for observational cohort and cross-sectional studies.11,12 For each study 14 items were assessed independently by two authors (CS, MP) to establish if risk of bias was absent or present or undeterminable. In addition, reviewers assigned each study an overall subjective rating of quality (low, fair, good).11,12 Discrepancies were resolved through consensus or with the final judgment of a third author (MV): the percentage of inter-rater agreement was recorded.
Data collection and analysisTwo investigators (CS, MP) independently conducted the search of the databases, screening all titles and/or abstracts based on the inclusion criteria. Abstracts and/or full-text papers of all potentially eligible studies were retrieved and a record was kept of all studies not meeting the inclusion criteria together with the reasons for their exclusion. The same investigators independently inserted the data of potentially eligible articles in a Microsoft Excel (2013 version, Microsoft, Redmond, WA) institutional database. At the end of this process a dedicated meeting was held in order to define the final list of articles to be evaluated. Disagreement between investigators about eligibility was resolved by discussion and consensus: if consensus could not be reached, a third investigator (MV) adjudicated the findings.
For each study, we recorded type, country, number of centres involved, setting, sample size, patients’ age, measures used, and whether or not exercise-induced desaturation (EID), or exercise or ADL induced symptoms were assessed. The performance of rehabilitation/physiotherapy programs was also recorded, if any. Among symptoms, we included all those symptoms measured during or at the end of exercise tests or during physical activity (e.g. ADL). Symptoms measured at rest or not related to physical activity (e.g. ageusia, headache, etc.) were not considered in this review. The effects of an intervention (if any) on these measures were beyond the scope of the study.
For each measurement, we recorded results (mean and SD or median and IQR). When available, the time between the disease onset (index event: positive swab, hospitalization or emergency department admission) and the first administration of the measure was recorded. For the four most used measures, we performed a quick literature search for predicted values and we compared them with the mean or median data reported in the included studies. No other quantitative analysis (e.g. of the scores obtained in the measurement scales) was carried out.
ResultsWe identified 156 potentially relevant articles. Thirty-one observational studies (8 cross-sectional), 1 RCT and 1 study protocol were eligible for the analysis (Fig. 1).
Quality of the studiesTable 1 shows the methodological quality of the studies. The inter-rater agreement of item definitions was very good: 94.2%. The overall quality was considered as low for 11 studies, fair for 14, and good for 6 studies. The most frequent motives for bias were the absence of assessor blinding and the missing justification of the sample size or power estimation.
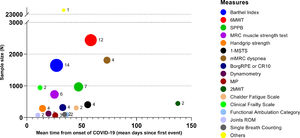
Characteristics of the studiesTable 2 shows the characteristics of the included studies. Most studies were from Europe, six from Asia,5,17,24,31,34,35 and one from the USA.27 The sample size of each study ranged from 14 to 20,889 participants, the mean or median age ranged from 49 to 72 years and in 13 out of 33 studies (39.4%) a rehabilitation program was performed. Twenty-eight measures were found, mostly administered in hospitalised subjects or during inpatient rehabilitation. Other settings were the emergency department (ED), ICU, and follow-up visits. Fig. 2 shows the proportion of studies using each measure of physical performance or of exercise or ADL induced symptoms, and the overall sample size of studies using each measure.
Principal characteristics of the 33 included studies. Quantitative data are expressed as mean ± SD or median (IQR).
| Reference | Country | Centres, n | Setting | PT/Rehab | Patients, n | Age, years | Measures used | EID assessment |
|---|---|---|---|---|---|---|---|---|
| Goodacre 13 | UK | 70 | EM Dept | N | 20,889 | 62.4 ± 19.7 | Performance status of the PRIEST COVID-19 Clinical Severity Score | N |
| McWilliams 14 | UK | 1 | ICU | Y | 110 | 53 ± 12 | Manchester Mobility Score, Clinical Frailty Scale | N |
| Ceriana 15 | Italy | 3 | Step-down unit (ICU) | N | 89 | 61.9 ± 11.3 | Barthel Index, MRC muscle strength test: quadriceps and biceps | N |
| Medrinal 16 | France | 2 | ICU | N | 23 | 66 ± 9 | MRC muscle strength test, MIP, ICU mobility scale | N |
| Tay 17 | Singapore | 1 | ICU | N | 51 | 56.3 ± 13.1 | Functional Ambulation Category | N |
| Van Aerde 18 | Germany | 1 | ICU | N | 486 | MRC muscle strength test, Barthel Index | N | |
| Ozyemisci Taskiran 19 | Turkey | 1 | ICU | Y | 14 | Handgrip strength, composite MRC muscle strength test, joints ROM | N | |
| Tuzum 20 | Turkey | 1 | Ward | N | 150 | 53.2 ± 15.5 | Handgrip strength, Chalder Fatigue Scale, motion induced pain | N |
| Paneroni 21 | Italy | 1 | Ward | N | 184 | 74 ± 12 | SPPB | N |
| Belli 22 | Italy | 1 | Ward | Y | 103 | 73.9 ± 12.9 | 1m-STS, SPPB, Barthel Index | N |
| Vilches-Moraga 23 | UK and Italy | 13 | Ward | N | 831 | 71 (58–81) | Clinical Frailty Scale | N |
| Zhu 24 | China | 28 | Ward | N | 432 | 49 (35–60) | Lawton's IADL scale, Barthel Index | N |
| Fuglebjerg 25 | Denmark | 1 | Ward | N | 26 | 63 (29–85) | 6MWT, Borg Dyspnoea after 6MWT | Y |
| Paneroni 4 | Italy | 1 | Ward | N | 41 | 67.1 ± 11.9 | 1m-STS, SPPB, Muscle dynamometry, Single-Breath Counting test, Borg Dyspnoea and fatigue after 1-MSTS and ADL | Y |
| Zampogna 26 | Italy | 1 | Ward | N | 56 | 69.4 ± 9.9 | Barthel Dyspnoea Index, Barthel Index, SPPB, MRC muscle strength test of quadriceps and biceps, Single Breath Counting, 6MWT, | |
| 1m-STS | N | |||||||
| Bowles 27 | The USA | 64 | Home hospital acute care | N | 1409 | 67 ± 15 | ADL dependency, dyspnoea during ADL, motion induced pain | N |
| Curci 28 | Italy | 1 | Inpatient Rehab | Y | 32 | 72.6 ± 10.9 | Barthel Index, mMRC dyspnoea, 6MWT | N |
| Wiertz 29 | Netherlands | 1 | Inpatient Rehab | N | 60 | 59.9 ± 10.2 | Barthel Index, MRC muscle strength test, dynamometry; joints ROM; fatigue and dyspnoea (numeric rating scale 0–10). | Y |
| Zampogna 30 | Italy | 4 | Inpatient Rehab | Y | 140 | 71 (61–78) | SPPB, Barthel Index, 6MWT | N |
| Sakai 31 | Japan | 1 | Inpatient Rehab | Y | 43 | 65 (21–95) | Barthel Index, ability to walk | N |
| Curci 32 | Italy | 1 | Inpatient Rehab | Y | 41 | 72.1 ± 11.1 | Barthel Index, mMRC dyspnoea, 6MWT, Borg RPE | N |
| Puchner 33 | Austria | 2 | Inpatient Rehab | Y | 23 | 57 ± 10 | 6MWT, Barthel Index, MIP | N |
| Liu 34 | China | 2 | Inpatient Rehab | Y | 72 | 69.1 ± 7.6 | 6MWT, FIM | N |
| Zhang 35 | China | 1 | Inpatient Rehab | Y | mMRC dyspnoea, Barthel Index, Patient Health Questionnaire-9 scale, Respiratory Symptoms scale | N | ||
| Piquet 36 | France | 1 | Inpatient Rehab | Y | 100 | 66 ± 22 | Barthel Index, 10-times sit-to-stand, Handgrip strength, Borg RPE | N |
| Al Chickanie 37 | France | 1 | Inpatient Rehab | Y | 21 | 70.9 ± 10.6 | MIP, MEP, Tinetti balance test, 6MWT, Handgrip strength, quadriceps dynamometry, Borg Dyspnoea | Y |
| Bertolucci 38 | Italy | 1 | Inpatient Rehab | Y | 39 | 67.8 ± 10.8 | Barthel Index, Functional Ambulation Category | N |
| Sonnweber et al. 39 | Austria | 1 | Home follow-up | N | 109 | 58 ± 14 | 6MWT | N |
| Townsend et al. 40 | Ireland | 1 | Home follow-up | N | 153 | 48 (35–59) | 6MWT, Borg Dyspnoea scale, Chalder Fatigue Scale | Y |
| Daher et al. 41 | Germany | 1 | Home follow-up | N | 33 | 64 ± 3 | 6MWT, Borg Dyspnoea and fatigue after 6MWT | N |
| Baricich et al. 42 | Italy | 1 | Home follow-up | N | 204 | 57.9 ± 12.8 | SPPB, 2MWT, 1m-STS | N |
| Bellan et al. 43 | Italy | 1 | Home follow-up | N | 238 | 61 (50–71) | SPPB, 2MWT | N |
| Huang et al. 5 | China | 1 | Home follow-up | N | 1733 | 57 (47–65) | mMRC dyspnoea, 6MWT | N |
Abbreviations: EID, Exercise Induced desaturation; n, number; PT/Rehab, Physiotherapy/Rehabilitation; EM, emergency; ICU, Intensive Care Unit; MRC, Medical Research Council; MIP, maximal inspiratory pressure; ROM, range of motion; IADL, instrumental activities of daily living; mMRC, modified Medical Research Council scale; ADL, activities of daily living; ATS/ERS, American Thoracic Society/European Respiratory Society; RPE, rate of perceived exertion; MEP, maximal expiratory pressure; SPPB, Short Physical Performance Battery; 6MWT, 6-min walking test; 2MWT, 2-min walking test; 1m-STS, 1-min sit-to-stand; FIM, Functional Independence Measure; Borg RPE, Borg Rating Perception of Exertion scale; d, days; Y, yes; N, no.
The Barthel Index44,45 (14 studies: 42.4%),15,18,22,24,26,28–33,35,36,38 Six-Minute Walking Distance Test (6MWT) 46 (12 studies: 36.4%),5,25,26,28,30,32–34,37,39–41 Short Physical Performance Battery (SPPB)47,48 (7 studies: 21.2%)4,21,22,26,30,42,43 and 1-Minute Sit-to-Stand (1m-STS)49,50 (4 studies: 12.1%)4,22,26,42 were the most used tests (Fig. 2). The Barthel Index was mainly used in the acute phase, whereas the 6MWT was assessed in interventional and follow-up studies. The SPPB was mainly used in the acute ward.
Table 3 shows sample sizes and results of the four most used measures of physical performance in the different settings. When comparing reported values with the reference values available in the literature, we found lower values for the Barthel Index (range of mean or median reported values vs “reference values”: 11–77 vs 100 points45), 1m-STS (11–22 vs 22–37 repetitions/min in people aged 75–79 years 51), SPPB (0.5–7.9 vs 11.4 ± 1.3 points 52), and 6MWT (45–223 vs 380–782 m 53) respectively.
Values of the most employed outcome measures in the 33 included studies (total population = 27,935 patients). Data are reported as mean ± SD or median (IQR).
| Reference | Setting | N | Mean ± SD Median (IQR) | |
|---|---|---|---|---|
| Barthel index | Ceriana15 | ICU | 70 | 27.7 ± 31.0 |
| Zampogna 30 | R | 140 | 55 (30–90) | |
| Sakai 31 | R | 43 | 75 (0–90) | |
| Curci 28 | R | 32 | 45.2 ± 27.6 | |
| Curci 32 | R | 41 | 43.4 ± 26 | |
| Puchner 33 | R | 23 | 83 ± 18 | |
| Piquet 36 | R | 100 | 77 ± 27 | |
| Wiertz 29 | R | 60 | 11 ± 6 | |
| Bertolucci 38 | R | 39 | 75 (0–100) | |
| SPPB | Paneroni 21 | Ward | 184 | 3.1 ± 3.9 |
| Paneroni 4 | Ward | 41 | 7.9 ± 3.3 | |
| Zampogna 26 | Ward | 56 | 0.5 (0–6) | |
| Zampogna 30 | R | 140 | 3.24 ± 3.69 | |
| Baricich 42 | Home | 204 | 11.2 ± 1.4 | |
| 1STS | Belli 22 | Ward | 43 | 14 ± 6 |
| Paneroni 4 | Ward | 41 | 22.1 ± 7.3 | |
| Zampogna 26 | Ward | 19 | 14 (9.3–19.8) | |
| Baricich 42 | Home | 204 | 19.7 ± 7.3 | |
| 6MWT | Zampogna 26 | Ward | 4 | 424 ± 35 |
| Curci 28 | R | 6 | 45 ± 101 | |
| Curci 32 | R | 6 | 240 ± 81 | |
| Puchner 33 | R | 23 | 323 ± 196 | |
| Liu 34 | R | 72 | 159 ± 77 | |
| Al Chickanie 35 | R | 21 | 139 ± 144 | |
| Zampogna 30 | R | 42 | 229 ± 102 | |
| Townsend40 | Home | 109 | 460 (225–640) | |
| Daher 41 | Home | 33 | 380 (180–470) | |
| Huang 5 | Home | 1733 | 495 (440–538) |
Abbreviations: N, number of patients; R, rehabilitation centre; SPPB, Short Physical Performance Battery; 1STS; 1-Min Sit-to-Stand; 6MWT, 6-Min Walking Test; SD, Standard Deviation; IQR, Interquartile Range.
Exercise-induced dyspnoea was assessed in twelve studies.4,5,9,25–29,35–37,40,41 The most commonly used scale to assess dyspnoea in daily life was the modified Medical Research Council (mMRC) scale54 used in four studies.5,28,32,35 Two studies in a rehabilitative setting found the most severe score (level 5) in 87.5 and 90.2% of patients.28,32 One study5 reported that, at six months following disease onset, 26% of patients had mMRC levels greater than 1. Only one study26 used the Barthel Dyspnoea Index55 in a rehabilitative setting, and reported moderate levels of dyspnoea during ADL. Exercise-induced dyspnoea was evaluated at the end of the 6MWT by the Borg scale56 in four out of twelve studies.25,37,40,41 One study4 assessed dyspnoea at the end of the 1m-STS. Two studies27,29 used other numeric scales to measure exercise-induced dyspnoea.
Fatigue was assessed in seven studies.4,20,29,32,36,40,41. Two studies20,41 used the Chalder Fatigue Scale, which is a dedicated tool to measure fatigue. Two other studies4,41 measured fatigue with the Borg scale at the end of the 6MWT, and two more studies23,36 measured the (Borg) Rate of Perceived Exertion. One study29 assessed fatigue using a 0–10 numeric rating scale. Motion induced pain was assessed in two studies.20,27
Exercise induced desaturationExercise-Induced Desaturation was reported in five studies.4,25,29,40,41 It was defined as oxygen saturation (SpO2) < 90% in four studies;25,29,40,41 in the other study,4 it was defined as a reduction in SpO2 by > 3 % points during the exercise tests. In the acute setting, 24–50% of patients demonstrated EID.4,25 One study29 in the rehabilitation setting reported EID in 38% of patients assessed.
RehabilitationThirteen studies14,19,22,28,30–38 included at least one rehabilitative intervention during the time-course of the study. Four studies30,31,37,38 described structured multidisciplinary rehabilitation programs, while in five studies14,19,23,31,36 the rehabilitation was a short intervention provided to respond to the needs of patients during the first phase of the pandemic. In two studies28,32 the components were selected according to the patient's level of oxygen saturation.
DiscussionIn this mapping review, we presented the measures of physical performance employed in studies on patients with COVID-19. In addition, we presented also the measures of dyspnoea and other exercise- or ADL-induced symptoms. In the studies evaluated, mostly of low or fair quality, we found twenty-eight measures used, the Barthel Index,44,45 6MWT,46,53 SPPB47,48,52 and 1m-STS49-51 being the ones most frequently used. . The other tests were reported in a few studies or even in just one. A wide range of impairment in physical performance (e.g. from 11% to 77% of normal values for Barthel Index) was reported with the use of these tools.
Patients recovering from COVID-19 may show impairment in respiratory function,57 and the majority of patients hospitalised with COVID-19 report persistent symptoms several months after infection onset.5,58 However studies evaluating symptoms may suffer from recall bias and subjective rating of symptoms. Therefore, tools that objectively measure the functional consequences of COVID-19 disease in the short- and long-term are necessary.
In routine clinical practice, the Barthel Index is the most widely used scale to measure patients’ motor and functional disabilities in ADL.45 This index was developed for chronic and long-term hospital patients with neurological diseases to examine their performance before and after treatment and predict the time needed for motor rehabilitation and the degree of nursing aid required.45
The 6MWT is the gold standard field exercise test and it has been validated for most chronic lung diseases. It is sensitive, reproducible, easy to perform, and does not require any specialized equipment.46
The SPPB represents the sum of the scores in three component tests of functional relevance, namely standing balance, 4-meter gait speed, and the five-repetition sit-to-stand test.47 The SPPB is the most commonly used performance-based measure for patients with chronic obstructive pulmonary disease (COPD). It is a standardized objective tool, rapid and simple to conduct, and less influenced by cultural and educational background than other self-reported measures. Because lower-limb strength is important for a satisfactory completion of the mobility activities, the SPPB has also been cited as a measure of lower-extremity function.59 It has also been shown that the SPPB is significantly related to the capacity to perform ADL, such as changing and maintaining body position, carrying, moving, and handling objects, or walking and gait pattern.47
The 1m-STS requires only a chair and is easy to perform, making it feasible for use in the physician's office.60 Studies to date have shown that the 1m-STS is well tolerated, sensitive, and reproducible in patients with COPD,49 cystic fibrosis61 and interstitial lung diseases.50
Dyspnoea is a symptom limiting exercise and ADL; therefore we searched the literature also for papers reporting this symptom. The severity of dyspnoea cannot be predicted from lung function; therefore, dyspnoea must be assessed specifically. Several instruments are commonly used to measure different domains of dyspnoea such as sensory-perceptual experience, affective distress, symptom impact or burden.62 We found twelve studies investigating dyspnoea during physical activity with various scales.
Fatigue is an important debilitating symptom affecting all chronic respiratory diseases. It is a leading cause of consultations with major clinical implications. Despite its well-acknowledged negative impact on the patient's life, fatigue is still a misunderstood and underdiagnosed symptom in respiratory diseases such as COPD. Consequently, there is currently no specific intervention to treat all aspects of this symptom which is rather often considered as a secondary outcome in interventions aiming primarily to increase physical fitness and/or health related quality of life.63 There is low-grade evidence of a positive effect of exercise training on perceived fatigue, at least in patients with COPD.64
Pain during motion is a debilitating symptom responsible for reduced functional performance. No dedicated scales were used to investigate this symptom, but two studies reported the presence/absence of pain during motion.20,27
Exercise induced desaturation is associated with exercise limitation. When evaluating individuals with EID a crucial point is the definition, which varies widely across clinical trials, ranging from SpO2 ≤ 88% to a decrease in SpO2 of ≥ 4% with or without a nadir SpO2 of < 90%.65–68
The results of our review confirm that patients with COVID-19 infection of differing severity suffer from a decline in physical performance in the short-4 and long-term.5 The wide range of results as shown by the SD or IQR reported in the studies and the differences in findings across settings indicates differences in case mix and times of evaluation. However, it should be born in mind that, particularly in the first wave of the pandemic, the allocation of patients might have been influenced by organisational issues, such as bed shortage in ICU or acute wards, over and above the patient's clinical conditions. The different values of physical performance reported with the different measures used confirm that these tools assess somewhat different aspects of physical performance and highlight the need for a more homogeneous set of tools to measure the outcome of these patients.
The quality of most of the studies was judged as fair or low; this result was expected. The sudden outbreak of the pandemic and the rapid need of information from the scientific community have led to a high index of publications,69 on the top of the overwhelming clinical pressure on researchers at the time which has resulted also in a higher level of retractions.70
This study has limitations. We conducted the search in a limited number of indexed databases, and keywords included dyspnoea but no other symptoms potentially relevant in physical performance tests. However, the most important limitation is the fact that the pandemic is still ongoing, which will result in increasing numbers of studies on the issue addressed. . However, we are confident that our search will contribute to those future studies (like in Heisenberg uncertainty principle).
ConclusionThis mapping review of measures used in COVID-19 patients shows studies mostly of low or fair quality, characterized by a large variability of measures, which overall indicate an impairment in physical performance. Our findings should be interpreted with caution. In fact, the studies were all, except one, observational with suboptimal methodological quality. Very different measures have been used which have different requirements (scale, availability of space…). Butthe choice of which measures to use according to the phase of the disease and setting of application is an issue that also need research on measurement properties in this population, which is still lacking. Better standardisation in the choice, timing and interpretation of measurement of physical performance is mandatory. Future RCTs or studies with higher methodological quality are required to clarify the validity of measures used in COVID-19 and in which setting, and verify the changes over time and/or in response to treatment.
Authors' contributionsCS and MP contributed to data acquisition and data analysis; all authors participated in drafting the article or critically revised it for important intellectual content. All authors contributed to the conception and design, data interpretation, final approval of the version to be published and agreed to be accountable for all aspects of the work and in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.