Patients with acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) may experience severe acute respiratory failure, even requiring ventilatory assistance. Physiological data on lung mechanics during these events are lacking.
MethodsPatients with AE-IPF admitted to Respiratory Intensive Care Unit to receive non-invasive ventilation (NIV) were retrospectively analyzed. Esophageal pressure swing (ΔPes) and respiratory mechanics before and after 2 hours of NIV were collected as primary outcome. The correlation between positive end-expiratory pressure (PEEP) levels and changes of in dynamic compliance (dynCRS) and PaO2/FiO2 ratio was assessed. Further, an exploratory comparison with a historical cohort of ARDS patients matched 1:1 by age, sequential organ failure assessment score, body mass index and PaO2/FiO2 level was performed.
ResultsAt baseline, AE-IPF patients presented a high respiratory drive activation with ΔPes = 27 (21–34) cmH2O, respiratory rate (RR) = 34 (30–39) bpm and minute ventilation (VE) = 21 (20–26) L/min. Two hours after NIV application, ΔPes, RR and VE values showed a significant reduction (16 [14–24] cmH2O, p<0.0001, 27 [25–30] bpm, p=0.001, and 18 [17–20] L/min, p=0.003, respectively) while no significant change was found in dynamic transpulmonary pressure, expiratory tidal volume (Vte), dynCRS and dynamic mechanical power. PEEP levels negatively correlated with PaO2/FiO2 ratio and dynCRS (r=–0.67, p=0.03 and r=–0.27, p=0.4, respectively). When compared to AE-IPF, ARDS patients presented lower baseline ΔPes, RR, VE and dynamic mechanical power. Differently from AE-IPF, in ARDS both Vte and dynCRS increased significantly following NIV (p=0.01 and p=0.004 respectively) with PEEP levels directly associated with PaO2/FiO2 ratio and dynCRS (r=0.24, p=0.5 and r=0.65, p=0.04, respectively).
ConclusionsIn this study, patients with AE-IPF showed a high inspiratory effort, whose intensity was reduced by NIV application without a significant improvement in respiratory mechanics. In an exploratory analysis, AE-IPF patients showed a different mechanical behavior under spontaneous unassisted and assisted breathing compared with ARDS patients of similar severity.
Idiopathic pulmonary fibrosis (IPF) is a life-threatening lung disease characterized by progressive deterioration of lung function and a median survival time of 3-5 years from diagnosis.1 Acute exacerbation of IPF (AE-IPF) leads to an acute deterioration of respiratory function, and severe hypoxemia, further worsening the prognosis.2 During these events, the typical usual interstitial pneumonia pattern (UIP) – the radiological and histological hallmark of IPF– overlaps with diffuse alveolar damage (DAD), sharing similarities with acute respiratory distress syndrome (ARDS) and often requiring respiratory support.3 Several studies show that. the need for mechanical ventilation (MV) is associated with high mortality4,5 in IPF patients. This is probably related to the pathophysiological properties of UIP-like fibrotic lung (i.e. collapsed induration) areas, elevated lung elastance, high inhomogeneity) that makes it more susceptible to ventilatory-induced lung injury (VILI).3,6
Based on a large number of clinical observations available in literature, and on some physiopathological speculations,3,7 we have theorized an elastic model with the aim of explaining the mechanical behavior of the fibrotic lung when subjected to positive end-expiratory pressure (PEEP) during invasive MV, namely the “squishy-ball” theory. According to this hypothesis, the application of PEEP on a UIP-like lung pattern can determine the protrusion of the more distensible areas through a dense anelastic fibrotic tissue circle. This causes increased rigidity and worse compliance, thus easing tissue breakdown. Despite lack of extensive evidence, we suggested considering MV only in selected cases of AE-IPF.3 In this scenario, non-invasive mechanical ventilation (NIV) may therefore represent an alternative option to assist these patients, although no specific recommendations have been made so far.3,8,9 In ARDS, the efficacy of NIV in reducing the patient's inspiratory effort early after application has been related to a favorable clinical outcome.10 Indeed, the mitigation of the respiratory drive might result in a lower risk of self-inflicted lung injury (SILI) during spontaneous breathing. SILI is very likely to worsen outcomes in patients undergoing acute respiratory failure (ARF).11
To the best of our knowledge there are still no available data on inspiratory effort and lung mechanics in patients with AE-IPF either during unassisted or assisted spontaneous breathing. The aims of this study were to explore inspiratory effort and respiratory mechanics, at baseline and 2 hours after NIV in AE-IPF patients and to compare the data with ARDS patients matched for clinical severity.
Materials and methodsStudy setting and designThis retrospective single center cohort study was carried out at the Respiratory Intensive Care Unit (RICU) of the University Hospitals of Modena (Italy) and conducted in accordance with the pre-existing Ethics Committee “Area Vasta Emilia Nord” approval (registered protocol number 348/18). Informed consent to participation in the study and permission for their clinical data to be analyzed and published were obtained from participants, as appropriate. For study purposes we further conducted a retrospective sub-analysis of data prospectively collected within a pre-registered clinical trial ClinicalTrial.gov (NCT03826797) and in accordance with the pre-existing Ethics Committee “Area Vasta Emilia Nord” approval (registered protocol number 266/16).
Study populationPatients with IPF developing an AE and consecutively admitted to the Respiratory Intensive Care Unit and to the Intensive Care Unit of the University Hospital of Modena over the period August 1st, 2016 to January 1th, 2022 were retrospectively considered eligible for enrollment.
Inclusion criteria were as follows: age >18 years; previously established diagnosis of IPF with a UIP pattern on a high resolution computed tomography (HRCT) scan; occurrence of acute exacerbation of IPF as defined by an acute, clinically significant respiratory deterioration characterized by evidence of new widespread alveolar abnormality on chest HRCT scan and presence of ARF with PaO2/FiO2 ratio <300 mmHg;12 having received a NIV trial while on RICU stay; inspiratory effort assessment and monitoring through esophageal manometry.
Patients were excluded if they presented any of the following: acute cardiogenic pulmonary edema, concomitant hypercapnic respiratory failure (PaCO2 >45 mmHg) of any etiology, neuromuscular disease or chest wall deformities, home long-term oxygen therapy, lack of core data (i.e. clinical characteristics at baseline and physiological measurement) in medical record analysis.
AE-IPF population was then matched 1:1 by age, PaO2/FiO2 ratio, body mass index (BMI), sequential organ failure assessment (SOFA) score, to a group of patients with ARDS under spontaneous breathing, extracted from our dataset and treated between 2016 and 2022. All patients underwent a common and standardized intervention (including esophageal pressure monitoring) and data were collected using a standard collection protocol. The values of PaO2/FiO2 ratio used for matching these groups were those measured immediately before starting NIV.
General measurementsMedical reports, electronical charts and available clinical and physiological datasets were investigated to collect data on demographics, clinical characteristics, arterial blood gases, PaO2/FiO2 ratio, respiratory rate (RR), blood lactate level, clinical severity (as assessed by the SOFA score on RICU admission), esophageal manometry and respiratory mechanics before and after NIV trial.
Physiological measurementsAccording to our local protocol, esophageal manometry was performed with a multifunctional nasogastric tube with a pressure transducer (NutriVentTM, SIDAM, Mirandola, Italy) connected to a dedicated monitoring system (OptiVentTM, SIDAM, Mirandola, Italy) recording swings in esophageal (Pes) and dynamic transpulmonary (PL) pressures. The NutriVent was placed before starting NIV as previously reported10 and according to (or following the) the recommended calibration protocol.13,14 In order to avoid using absolute values for Pes and PL, we always referred to ΔPes and ΔPL from the end-expiratory level, respectively, calculated as recommended.15 For all the measurements, the beginning of the inspiratory phase was identified at the instant of Pes initial decay while the end of inspiration was considered to be the value of Pes where 25% of the time had elapsed from maximum deflection to baseline (eFigure 1, Supplementary Materials). The respiratory flow was measured through an external heated pneumotachograph (Fleisch No.2, Lausanne, Switzerland) inserted between the patient's oronasal facemask (BluestarTM, KOO Medical Equipment, Shanghai, PRC) and a connector with a side port for measurements. Expiratory tidal volume (Vte) was obtained by numerical integration of the flow signal; Vte was then adjusted to the predicted body weight (PBW) to derive Vte/kg of PBW. Minute ventilation (VE) was calculated as the product of Vte and RR. Vte/ΔPL was measured as a surrogate for respiratory system compliance and named “dynamic compliance” (dynCRS). Air Leaks from the oronasal facemask were computed using dedicated ventilator-integrated software (GE Healthcare Engstrom CarestationTM, GE Healthcare, Finland) based on the equation: leaks (L/min) = (inspiratory Vt – expiratory Vt) x RR. A surrogate of mechanical power (i.e. “dynamic mechanical power”) was then calculated as 0.098 * RR * Vte * (ΔPL + Positive end-expiratory pressure [PEEP]).15 In every patient of both groups, measurements were recorded under standardized conditions over five consecutive minutes of unassisted spontaneous breathing, and repeated 2-hours after the initiation of NIV. Data were numerically stored and downloaded from a USB stick at each time point.
NIV trialAccording to our local protocol, patients treatment was escalated to a trial of NIV if deemed indicated by the attending clinician, blinded to the study purposes and physiological measurements. The criteria to upgrade to NIV included PaO2/FiO2 ratio below 100 mmHg and/or RR > 30 bpm and/or persistence of respiratory distress and dyspnea despite HFNC set at 60 L/min. NIV was started and set by a skilled respiratory physician. Patients were connected to a conventional circuit via an appropriately sized oronasal facemask equipped with a dedicated output for probes (BluestarTM, KOO Medical Equipment, Shanghai, PRC) to a high-performance ventilator (GE Healthcare Engstrom CarestationTM, GE Healthcare, Finland) set in pressure support mode. A heat and moisture exchanger (HME) (Hygrobac, DAR, Mirandola, Italy) was inserted into the ventilator circuit's Y-piece. The delivered FiO2 was adjusted to target a SpO2 of 88–94%. None of the patients received any kind of sedation under NIV treatment. PEEP was initially set at 6 cmH2O and subsequently fine-tuned to target a peripheral oxygen saturation (SpO2) >92% with a delivered inspiratory fraction of oxygen (FiO2) less than 0.7. Pressure support (PS) was increased from 10 cmH2O, according to tidal volume (Vte/kg of body weight predicted-PBW), to target a Vte/kg <9.5 mL/kg of PBW16 and a RR <30 breaths/min. The inspiratory trigger and respiratory cycling were set at 3 L/min and at 25% of the inspiratory peak flow, respectively. An oronasal fitted mask was tightened to target a leak flow lower than 20 L/min. According to our local protocol, after 2 hours of NIV patients were re-assessed on a clinical and physiological basis.
Analysis planThe primary aim of the study was to explore inspiratory effort and respiratory mechanics, at baseline and 2-h following NIV application, in patients with AE-IPF. Data were displayed as median and IQR (interquartile range) for continuous variables and numbers and percentages for dichotomous variables. The paired Student's t-test assessed the difference between variables before and after NIV application, when distributed normally; otherwise, the Wilcoxon test was used. The relationship between PEEP and relative change in dynCRS and PaO2/FiO2 ratio 2 hours after starting NIV was tested with the Pearson correlation coefficient and assessed through linear regression. As an exploratory analysis, we compared the mechanical variables of AE-IPF patients with an ARDS population extracted from our dataset and including patients, studied by our group between 2016 and 2022. The ARDS comparison cohort was built using a one-to-one propensity score matching procedure with the nearest-neighbor method without replacement. The logit of the score was taken with a caliper of 0.2 in order to maximize the number of patients without comprising the match. Comparison between continuous variables was performed with Student's t-test distributed normally; otherwise, the Wilcoxon test was used. Dichotomous variables were compared using the χ2 test or Fisher's exact test, where appropriate. ANOVA and Kruskal-Wallis were used to test an interaction for whether the change in physiological variables 2 hours after NIV were different between groups. Statistics was performed using SPSS version 25.0 with PSMATCHING3 R Extension command (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 8.0 (GraphPad Software, Inc., La Jolla, Ca, USA) unless otherwise indicated.
ResultsClinical features of study populationThe flowchart of this study is shown in Fig. 1. Over the study period a total of 48 patients with AE-IPF were eligible for enrollment. Of these, 10 patients were analyzed. All of them were diagnosed with IPF based on the presence of a definite UIP pattern on HRCT scan. Patients were predominately male (7/10) with a median age of 75 years (65–78) (Table 1). The median value of clinical severity scores was 2 (2 – 2), 12.5 (9.8 – 21) and 30.5 (29 – 37.5) for SOFA, APACHE II and SAPS II scores respectively. The median time interval between IPF diagnosis and AE-IPF onset was 25 (15–33) months, while time lapse from hospital admission to NIV upgrade while in AE was 12 (7.5–27) hours. All patients died as inpatients.
General and clinical characteristics in the study groups on admission.
| Parameter | AE-IPF | ARDS | p value |
|---|---|---|---|
| Number of patients | 10 | 10 | |
| Age, years | 75 (65–78) | 75 (65–78) | 0.9 |
| Male, n | 7 (70) | 7 (70) | 0.9 |
| BMI, kg/m2 | 23 (21–25) | 23 (21–25) | 0.9 |
| Charlson index, score | 3 (3–5) | 4 (3–5) | 0.9 |
| Kelly scale, score | 1 (1–1) | 1 (1–1) | 0.9 |
| SOFA, score | 2 (2–2) | 2 (2–2.5) | 0.9 |
| APACHE, score | 12.5 (9.8–21) | 11 (10–21) | 0.9 |
| SAPS II, score | 30.5 (29–37.5) | 33 (32–38) | 0.8 |
| HACOR, score | 7 (6–8) | 5.5 (5–6) | 0.01 |
| †PaO2/FiO2, mmHg | 108 (80–126) | 105 (83–125) | 0.9 |
| †pH, value | 7.49 (7.47–7.52) | 7.48 (7.44–7.5) | 0.9 |
| †PaCO2, mmHg | 31 (28–32) | 33 (29–34) | 0.3 |
| Blood lactate, mmol/L | 1.2 (1–1.9) | 1 (0.9–1.2) | 0.1 |
| Serum creatinine, mg/dL | 0.9 (0.7–1.4) | 0.7 (0.6–1.4) | 0.9 |
| *PEEP, cmH2O | 6 (5.5–6.5) | 7 (6–8) | 0.1 |
| *PSV, cmH2O | 12 (10–12) | 12 (10–12.5) | 0.7 |
Data are presented as number (n) and percentage for dichotomous values or median and interquartile ranges (IQR) for continuous values.
The values of PaO2/FiO2 ratio used for matching these groups as well as pH and PCO2 values were those measured during high-flow nasal oxygen immediately before starting NIV.
AE-IPF = acute exacerbation of IPF; ARDS = acute respiratory distress syndrome; BMI = body mass index; HACOR = heart rate, acidosis, consciousness, oxygenation, respiratory rate; SOFA = sequential organ failure assessment; APACHE II = Acute Physiology and Chronic Health Evaluation II; SAPS II = Simplified Acute Physiology Score; PEEP = positive end-expiratory pressure; PSV = pressure support; IQR = interquartile range
Respiratory mechanics of IPF before and after 2 hours of NIV are shown in Table 2. During unassisted breathing, IPF patients displayed a median value of ΔPes (ΔPL) of 27 (21 – 34) cmH2O and a RR of 34 bpm. DynCrs was 28 mL/cmH2O while dynamic mechanical power was 71 J/min. After 2 hours of NIV, ΔPes was significantly reduced (16 [14 – 24] cmH2O, p<0.0001). Similarly, NIV application lowered both RR and VE (27 [25 – 30], p 0.001 bpm and 18 [17 – 20] L/min, p.003, respectively) while ΔPL, Vte, dynCRS and dynamic mechanical power did not change significantly.
Physiological variables of the AE-IPF population at baseline and 2 hours apart of NIV.
Data are presented as median value and interquartile range.
AE-IPF = Acute exacerbation of IPF; bpm = breaths per minute; IQR = interquartile range; NIV, non-invasive mechanical ventilation; ΔPes, change in esophageal pressure; ΔPL, change in dynamic transpulmonary pressure; RR, respiratory rate; VE, minute ventilation; Vte, expiratory tidal volume; DynCRS = dynamic respiratory system compliance; PBW = predicted body weight.
Mechanical power = 0,098 * (ΔPL + PEEP) * Vte * RR.
In AE-IPF patients, after two-hours of NIV, PEEP levels were significantly inversely correlated with PaO2/FiO2 ratio (r=–0.67, p=0.03, Fig. 2, panel A). Similarly, an inverse correlation between PEEP levels and dynCRS variation was observed (r=-0.27, p=0.4, Fig. 2, panel B), although statistical significance was not reached.
Correlation between PEEP values and change in both PaO2/FiO2 and dynCRS ratio in AE-IPF (panel A and B, respectively). PEEP levels were inversely correlated with both PaO2/FiO2 ratio (r=–0.67, p=0.03) and dynCRS (r=–0.27, p=0.4) in AE-IPF, although statistical significance was not achieved for the latter. AE-IPF = Acute exacerbation of IPF; ARDS = acute respiratory distress syndrome; dynCRS = respiratory system compliance; PEEP = positive end-expiratory pressure.
AE-IPF and matched ARDS groups were similar for clinical severity scores (APACHE and SAPS II, Table 1) at inclusion. Further, no group differences were found between pressure values set during the NIV trial (Table 1). All ARDS patients presented a pulmonary ARDS occurring from lung infection (4 viral, 4 bacterial and 2 pneumocystosis).
Before starting NIV, ARDS patients showed a lower ΔPes as compared to matched AE-IPF patients (24 [22 – 28] cmH2O, p=0.004). Similarly, ARDS group showed a lower baseline RR (27 [26 – 30] bpm, p=0.0004), VE (18 [17 – 21] L/min, p=0.04) and dynamic mechanical power (48 [52 – 62] J/min, p=0.01). Conversely, ARDS patients showed comparable values of baseline Vte (9.9 [9.6 – 11] mL/kg of PBW, p=0.1) and baseline dynCRS (28 [25 – 33] mL/cmH2O, p=0.3).
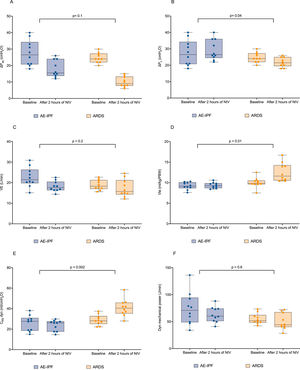
Compared to AE-IPF, patients with ARDS still presented a lower median value of RR, ΔPes, ΔPL, and dynamic mechanical power 2-hours after the initiation of NIV (21 [18 – 22] bpm, p<0.0001, 9 [8 – 13] cmH2O, p=0.001, 21.5 [19.5 – 25] cmH2O, p=0.003, 44 [40 – 68] J/min, p=0.01). At that time point both Vte and dynCRS were significantly higher in ARDS as compared to AE-IPF (11.6 [11 –14.2] cmH2O, p=0.001 and 41 [35 –46] mL/cmH2O, p=0.001 respectively). Two-hours after starting NIV and differently from AE-IPF, ARDS patients showed a direct correlation between PEEP levels and dynCRS (r=0.65, p=0.04, eFigure 2, panel A), while no direct association was found with PaO2/FiO2 ratio (r=0.24, p=0.5, eFigure 2, panel B). When testing whether there was a difference between groups concerning the change in physiological variables 2-hour after NIV, ΔPL displayed an opposite response to NIV, being increased in AE-IPF and reduced in ARDS (p=0.04, Fig. 3, panel B). Vte, and dynCRS increased following NIV application in the ARDS cohort as compared to AE-IPF, (p=0.01 and p=0.002 respectively, Fig. 3 panel D and E), whereas no significant change in ΔPes, VE and dynamic mechanical power was found (Fig. 3, panel A, C, and F).
Measured individual values of ΔPes, ΔPL, VE, Vte, dynCRS and dynamic mechanical power in the matched study groups both at baseline and 2-hour after initiating NIV. When testing as an interaction for whether the change in physiological variables 2 hours after starting NIV was different between AE-IPF and ARDS, statistical difference was found for ΔPL (panel B, p=0.04), Vte (panel D, p=0.01) and dynCRS (panel E, p=0.002). AE-IPF = Acute exacerbation of IPF; ARDS = acute respiratory distress syndrome; NIV = non-invasive mechanical ventilation; ΔPes = esophageal pressure swing; ΔPL = dynamic transpulmonary pressure; VE = minute ventilation; Vte = expiratory tidal volume; dynCRS = respiratory system compliance.
To the best of our knowledge this is the first study that quantifies the inspiratory effort and explores the respiratory mechanics in patients with AE-IPF under spontaneous unassisted and noninvasive assisted breathing. Overall, when compared to ARDS, patients with AE-IPF: 1) report a peculiar increase in inspiratory effort which reflects a high activation of the respiratory drive during unassisted breathing; 2) reduce effort and respiratory frequency but not transpulmonary pressure under short-term NIV without any improvement in dynamic compliance; 3) show a detrimental effect of increased values of external PEEP to PaO2/FiO2 ratio and dynCRS. All these issues deserve discussion.
First, keeping spontaneous breathing preserved may have several potential benefits in patients with ARF including the avoidance of sedation and/or use of myorelaxants, the prevention of muscle mass loss, the spare of diaphragmic function, and the risk reduction for delirium onset. This seems even more important in AE-IPF for which the upgrade to invasive MV is often burdened by a high risk of VILI with unfavorable outcomes.4,5,17 However, a growing body of evidence has strengthened the hypothesis that the presence of intense respiratory effort during ARF plays a critical role in promoting SILI10,18-20 and unfavorable ventilatory outcomes.10 In patients with IPF, specifically, the excessive inspiratory effort may be even more detrimental as fibrotic lungs are a patchwork of different tissue elasticities.7 Thus, during spontaneous breathing, pleural pressure swing distribution is even more inhomogeneous and lung tissue deformation occurs unevenly; some lung areas being subjected to harmful level of stress/strain.21 In our cohort, the baseline value of inspiratory effort of AE-IPF was 27 cmH2O, as quantified by the esophageal manometry; the inspiratory effort was even higher than that reported in matched patients with ARDS. Similarly, baseline RR became more elevated in AE-IPF than in ARDS. Although it is difficult to give reasons for the difference observed in the activation of respiratory drive, we can speculate that dynCRS may not be fully representative of the regional lung stretch, the fibrotic lung being subjected to anisotropic behavior during inflation.7 In line with this, we could hypothesize that the physical stimuli derived from micro-strain could act as a mechanical input in the hyperactivation of respiratory drive of AE-IPF patients.22 Furthermore, given that the baseline dynCRS was similar between groups, one could speculate that factors other than gas exchange impairment might have boosted the respiratory drive of IPF patients, namely lung inflammation.23
Secondly as expected, and similarly to ARDS patients, NIV was effective in reducing both inspiratory effort and RR in AE-IPF. However, the change in respiratory mechanics was different between the two types of patients. Indeed, dynCRS displayed a significant improvement following NIV in ARDS but not in AE-IPF. Moreover, the values of dynamic transpulmonary pressure resulted persistently high in AE-IPF patients 2 hours following NIV application, thus suggesting a less favorable interaction with the ventilatory assistance.
Third, values of external PEEP applied by NIV were inversely correlated with PaO2/FiO2 ratio and dynCRS at 2-hours in AE-IPF, at difference with ARDS patients. These data suggest that the response to PEEP might reflect a different lung recruitability in AE-IPF and ARDS. Nevertheless, it was shown that a negative end-expiratory transpulmonary pressure can be reverted by incrementing PEEP values during controlled MV.7 These findings may suggest that fibrotic lungs also exhibit end-expiratory de-recruitment in the dependent lung zones during an acute exacerbation. However, PEEP titration to a positive end expiratory transpulmonary pressure seemed to worsen all the static respiratory measurements (namely driving pressure, lung compliance, end-inspiratory transpulmonary pressure). We do believe that the PEEP-induced mechanical derangement in the fibrotic lung may be explained by the “squishy ball effect”: high PEEP set to keep alveolar units open during expiration hyper-inflates recruitable lung areas through the anelastic surrounding zones, thus enhancing the end-inspiratory transpulmonary pressure effect, and exposing the lung at risk of substantial injury. The dynamic mechanical response exhibited by our patients mirrors the one reported in intubated and mechanically ventilated ones, thus suggesting that the UIP-like fibrotic lung displays a “squishy ball” behavior even with PEEP applied during spontaneous breathing. In this scenario, and given the detrimental effect of PEEP on the hyper-inflation of the recruitable lung zones, a mild sedation intended to lower the RR might be suggested to lower the RR and thus allowing for prolonged expiratory time.
The major strength of the study is the detailed and comprehensive measurement of respiratory effort and mechanics in a cohort of AE-IPF using a standard relatively invasive procedure (esophageal manometry). To our knowledge, these are the first data collected in this regard. Further, the standardized protocol for physiological variables collection applied at our center allows consistent measurements during esophageal manometry. Finally, the presence of a UIP pattern in all patients strengthens the homogeneity of mechanical data.
Our study also has several limits. First, the retrospective design and the reduced sample do only provide a preliminary pathophysiological insight in this condition. However, given that IPF is a rare condition, and that esophageal manometry can be extremely difficult to manage during an acute exacerbation of the disease, we are confident that these data might contribute to a better understanding of the mechanical behavior of fibrotic lung in spontaneous breathing. Second, the lack of a qualitative analysis of radiological images (namely the proportion of hyper-inflated lung tissue24,25) during exacerbations may weaken the interpretation of results. Third, a further comparison of AE-IPF patients under HFNC might have contributed to better specify the potential detrimental effect of NIV on the respiratory mechanics in these patients. Fourth, although the cohorts were matched according to clinical, mechanical and oxygenation criteria, the small sample size of the ARDS cohort may not reflect the heterogenous features of this population. Indeed, a more homogenous ARDS population could have improved the quality of the matching. Finally, given the retrospective nature of the study, NIV settings were decided by the attending physician, neither we did assess any specific local nor systemic biomarkers of inflammation (i.e. cytokines) that might have contributed to understand the role of disease severity on the respiratory drive.
ConclusionsIn this physiologic, preliminary retrospective study, spontaneously breathing patients with IPF showed an elevated inspiratory effort while on acute exacerbation of the disease. The application of NIV with an external PEEP was effective in reducing their respiratory drive but at the cost of deteriorating mechanics. Additional prospective studies with a larger sample size are required to further define the local mechanical consequences and even the local or systemic biological features in patients with fibrotic lungs during assisted and non-assisted spontaneous breathing. More research is also needed to focus on the different mechanical behavior of AE-IPF compared with ARDS of similar severity.
DeclarationsEthics approval and consent to participateThis study was conducted in accordance with the pre-existing Ethics Committee “Area Vasta Emilia Nord” approval (registered protocol number 348/18). Informed consent to participate in the study and to allow their clinical data to be analyzed and published were obtained from participants, as appropriate. For study purposes we further conducted a retrospective sub-analysis of data prospectively collected within a pre-registered clinical trial ClinicalTrial.gov (NCT03826797) and in accordance with the pre-existing Ethics Committee “Area Vasta Emilia Nord” approval (registered protocol number 266/16).
Consent for publicationConsent for publication was obtained by all patients enrolled, as appropriate.
Availability of data and materialsData are available at the Respiratory Disease Unit of the University Hospital of Modena, Italy, upon request.
FundingNo funding available.
Author contributionsRT, IC and AC designed the study, enrolled the patients, analyzed the data, and wrote the paper and should be considered as first authors. LT, RF, DA, FG, GB, LM, A Moretti and CC made substantial contributions to the literature review, data collection, and paper writing. CN, EB, SC, VS, SB and MG reviewed the literature, wrote the manuscript and produced the figures. A Marchioni and EC designed the study, wrote, reviewed and edited the manuscript and share senior authorship. All authors have read and approved the final version of the manuscript. RT, IC and AC share first authorship. AM and EC share senior authorship.