To evaluate the prognostic utility of inflammation-based prognostic scores in patients with ALK-positive metastatic or non-resectable non-small-cell lung cancer (NSCLC) treated with crizotinib.
Patients and MethodsA total of 82 patients with ALK-positive metastatic or non-resectable NSCLC who received ALK TKI crizotinib were included. Pre-treatment modified Glasgow prognostic score (mGPS), prognostic nutritional index (PNI), and systemic immune-inflammation index (SII) were calculated. Multivariable logistic regression and Cox proportional hazards models were used to assess the impact of pretreatment mGPS, PNI, and SII on overall survival (OS), progression-free survival (PFS), and objective response rate (ORR).
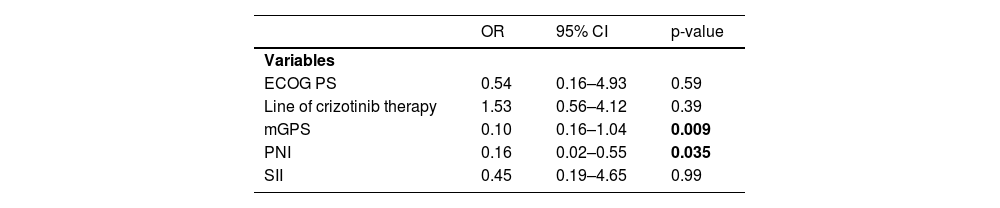
ResultsThe ORR was 77.2%, while 1-year OS and PFS rates were 95.0% and 93.5%, respectively. The univariate analysis revealed significantly higher 1-year PFS (89.4 vs. 64.4%, p=0.043) and OS (92.0 vs. 83.3%, p=0.01) rates in patients with low (<934.7) vs. high (≥934.7) SII scores. Multivariate analysis revealed that PNI ≥0.09 was a significant determinant of poorer 1-year OS rates (hazard ratio [HR]: 2.46, 95% confidence interval [CI] 0.88–4.85, p=0.035). No significant difference was observed in survival rates according to gender, age, smoking status, prior lines of therapy, or mGPS scores, while higher mGPS scores (odds ratio [OR]: 0.1, 95%CI 0.16–1.04; p=0.009) and higher PNI scores (OR: 0.16, 95% CI 0.02–0.55; p=0.035) were associated with poorer ORR.
ConclusionOur findings indicate the prognostic significance of PNI and SII in terms of survival outcome and the impact of mGPS and PNI on treatment response in patients with ALK-positive NSCLC treated with crizotinib.
Lung cancer remains among the most frequent and aggressive malignancies and the leading causes of cancer-related mortality worldwide.1,2 Non-small lung cancer (NSCLC) accounts for 85% of all lung cancers.3-5 The management of NSCLC has evolved over years from the traditional platinum-based chemotherapy to personalized therapy with anti-angiogenic agents or tyrosine kinase inhibitors (TKIs) in patients with targetable oncogenic driver alterations.6-9
Rearrangement of anaplastic large-cell lymphoma kinase (ALK) gene occurs in up to 7% of patients with NSCLC, particularly in younger and never or ex-light smoker patients with adenocarcinoma histology.9-13ALK-rearranged NSCLC tends to be more aggressive and usually presents at an advanced stage, where brain metastases are very common and pose significant clinical challenges.14,15
Crizotinib, a first generation ALK TKI, has been used as the first-line treatment for patients with ALK-positive NSCLC because of its superior efficacy over chemotherapy in terms of response rate and survival in stage IIIA-IV patients in PROFILE 1014 phase III randomized trials.8,16,17 However, crizotinib offers only a median progression-free survival (PFS) of 7.7-11 months.9,12 Moreover, despite the introduction of more selective second-generation (ceritinib, alectinib, and brigatinib) and third-generation (lorlatinib) ALK TKIs, approximately 30% of patients do not respond to ALK‐TKIs even if they harbor ALK rearrangement.18,19 Hence, given the aggressive course of the ALK-positive NSCLC along with the high treatment costs, potential severe toxicity, and risk of early progression in case of ineffective treatment, it is worthwhile to establish reliable prognostic markers to predict the efficacy of ALK-TKIs.
Systemic inflammation plays a critical role in proliferation, apoptosis, migration, invasion, and metastasis during carcinogenesis and cancer progression. Activated transcription factors prompt tumor cells to produce chemokines, cytokines, prostaglandins, and growth factors to recruit inflammatory cells including neutrophils, lymphocytes, macrophage, and mast cells.20 Systemic inflammatory responses play important role in tumor development, enhanced local immunosuppression and thereby in cancer progression and worsened prognosis. Therefore, prognostic value of several inflammation-based scores such as Glasgow prognostic score (GPS), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), prognostic nutritional index (PNI), systemic immune-inflammation index (SII) and advanced lung cancer inflammation index (ALI) have become increasingly investigated in NSCLC.21-28
The modified GPS (mGPS) is based on a combination of serum albumin level and C-reactive protein (CRP);29 the PNI is based on a combination of serum albumin level and absolute lymphocyte count,30 and the SII is based on a combination of NLR and platelet count.31 While the prognostic significance of GPS, PNI, or SII has been addressed in small cell lung cancer,29,32 stage III NSCLC,33 and resectable NSCLC,27,34 its value in patients with ALK-positive NSCLC remains unclear.
This study aimed to retrospectively evaluate the prognostic utility of pre-treatment mGPS, PNI, and SII scores in terms of treatment response and survival outcomes in patients with ALK-positive metastatic or non-resectable NSCLC treated with crizotinib.
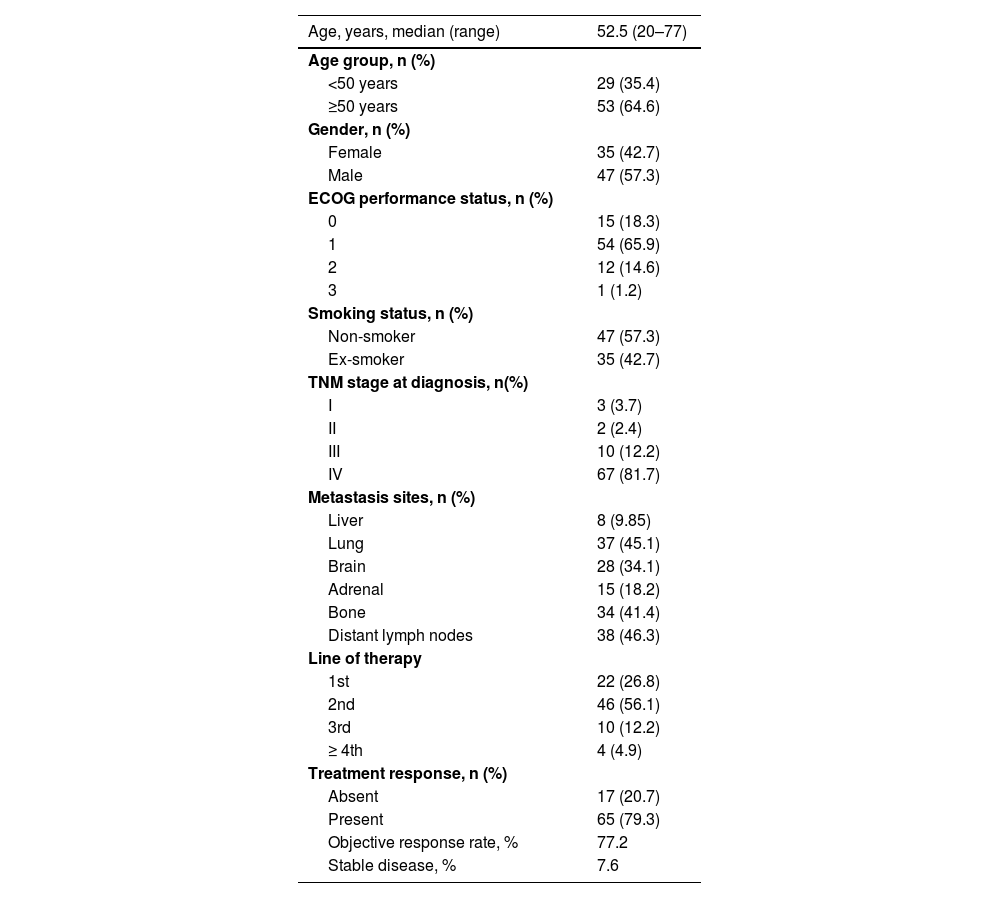
Patients and methodsStudy populationA total of 82 patients with median age of 52.5 (range, 20–77) years (57.3% males) who were diagnosed with metastatic or non-resectable ALK-positive NSCLC and received ALK TKI crizotinib between January 2013 and August 2019 were included in this retrospective multicenter study conducted in eight oncology centers across Turkey. Patients who had a concomitant infection including human immunodeficiency virus or hepatitis, systemic steroids, concomitant radiotherapy, or previous or ongoing autoimmune disorder were excluded.
The study was conducted in accordance with the ethical principles stated in the “Declaration of Helsinki” and approved by the institutional ethics committee along with the permission for the use of patient data for publication purposes (Date of Approval: 11 May 2022 Reference number/Protocol No: 449).
Study parametersData on patient demographics (gender, age), smoking history, Eastern Cooperative Oncology Group Performance Status (ECOG PS), disease characteristics (TNM stage at diagnosis, metastasis sites), laboratory findings within a week prior to the first dose of crizotinib (white blood cells, hemoglobin, neutrophil, platelet, and lymphocyte counts, albumin level and CRP), line of crizotinib therapy as well as follow-up data on treatment response were retrieved from the hospital records. Pre-treatment inflammation-based prognostic scores included mGPS, PNI, and SII.
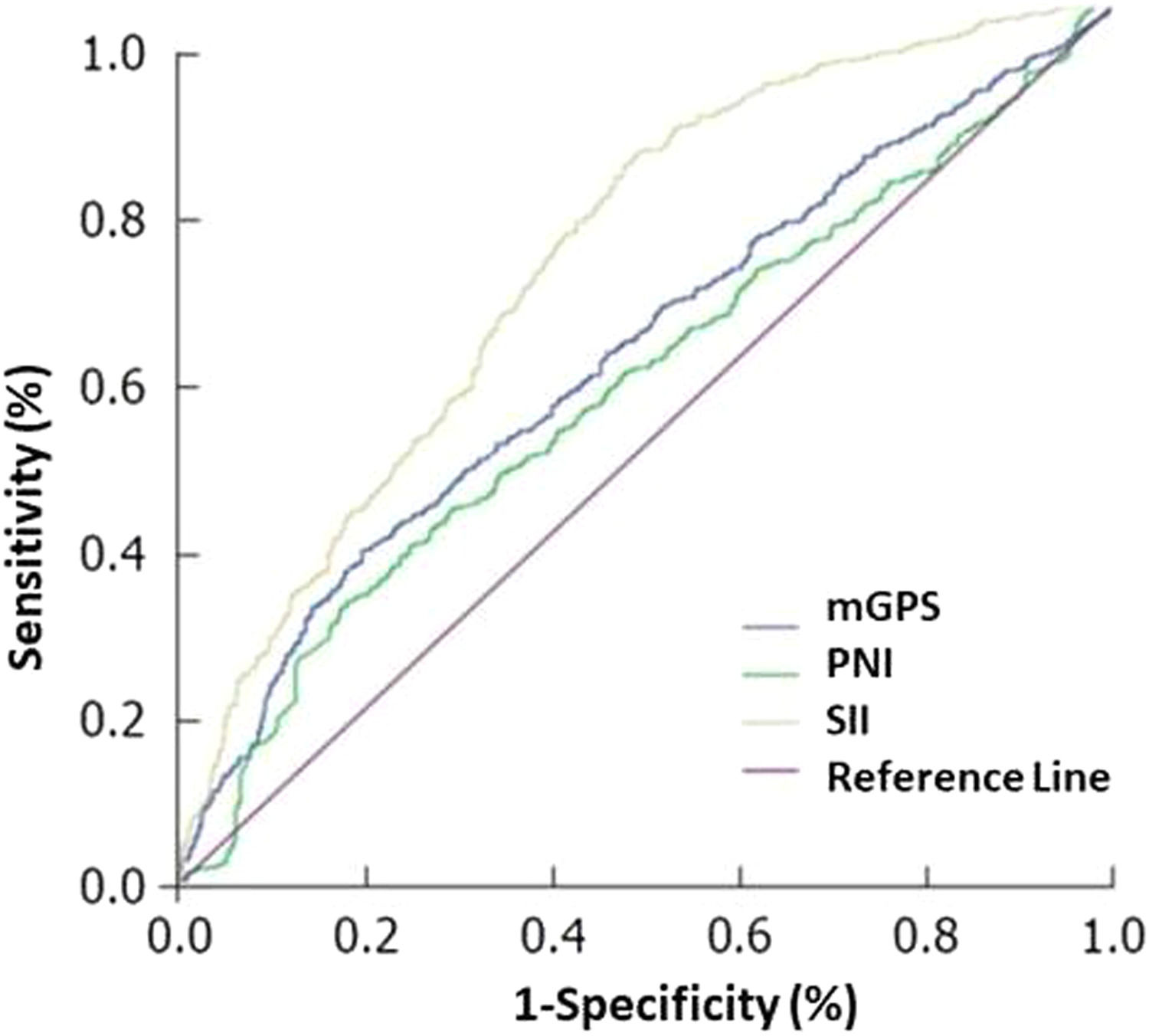
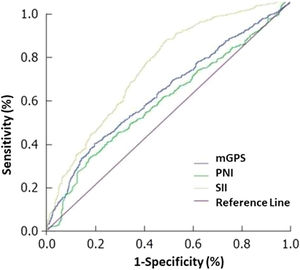
Survival analysis included 1-year overall survival (OS) and PFS rates and were evaluated with respect to clinicopathological variables and inflammation-based prognostic scores. For inflammation-based prognostic scores, receiver operating characteristic (ROC) analysis was performed to determine optimal cut-off values for mGPS, PNI, and SII; and the impact of inflammation-based prognostic scores on survival parameters were analyzed in subgroups of patients (lower vs. higher score) based on the optimum cut-off value defined for each score (Fig. 1).
Receiver operating characteristic (ROC) curve shows the cut-off value of inflammation-based prognostic scores indicative of response to crizotinib. According to ROC analysis, the areas under the curve for mGPS, PNI and SII are 0.859, 0.755 and 0.745, respectively, and the optimal cut-off points are 0.09, 0.09 and 934.7, respectively.
Multivariable logistic regression and Cox proportional hazards models were used to assess the impact of clinicopathological variables and inflammation-based prognostic factors on OS, PFS and objective response rate (ORR).
ALK rearrangement was determined using fluorescence in situ hybridization and Ventana immunohistochemistry (IHC). Tumor stage was classified according to the 8th edition of AJCC Cancer Staging Manual.35
Treatment response assessmentComputed tomography (CT)/magnetic resonance imaging scan examinations were performed every 8 weeks. Treatment response was evaluated based on Response Evaluation Criteria of Solid Tumor (RECIST) ver.1.136 and categorized as progressive disease, stable disease (SD), partial remission (PR) and complete remission (CR). ORR was defined as the percentage of the best overall remission (PR + CR) confirmed by the investigator.
Inflammation-based prognostic scoresmGPS was based on combined evaluation of elevated CRP and hypoalbuminemia and scored by 0 (normal CRP and albumin levels), 1 (CRP >1.0 mg/dL only), or 2 (CRP was >1.0 mg/dL plus albumin <3.5 g/dL).29 PNI was calculated according to the previously described formula: 10 × serum albumin value (g/ dL) + 0.005 × peripheral lymphocyte count (/μL).30 SII was calculated using the formula: platelet count × neutrophil count / lymphocyte count.31
Statistical analysisStatistical analysis was made using R 3.4.3 and IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY). Descriptive analysis was used for all variables. The survival outcomes measured were PFS and OS. PFS was defined as the period from the date of treatment to first recurrence or last follow-up and OS was defined as the period from the date of treatment to death or last follow-up. The optimal cut-off values of mGPS, PNI and SII for prognostic ability were determined according to ROC curve and the areas under the ROC curve. Patients were divided into high and low mGPS, PNI and SII groups based on cut-off values. PFS and OS rates were calculated and compared using the Kaplan‐Meier method and the log‐rank test. Univariate and multivariate analyses were performed for prognostic factors with inclusion of variables significant on univariate analysis to a multivariate Cox proportional hazards model. Hazard ratios (HRs) were estimated using the Cox analysis and reported as relative risks with corresponding 95% confidence intervals (CIs). Reverse Kaplan‐Meier method was used to compute the median follow‐up time. Data were expressed as median (minimum-maximum) and percent (%) where appropriate. P < 0.05 was considered statistically significant.
ResultsBaseline characteristics and treatment responseMedian age was 52.5 years (range, 20–77), and 57.3% of patients were males. Overall, 57.3% of patients were non-smokers and 65.9% were in ECOG PS category 1. Tumor characteristics at the time of diagnosis included TNM Stage IV disease in 81.7% of patients along with metastasis to distant lymph nodes in 46.3%. Overall, 26.8% of patients were treated with first-line of crizotinib therapy and 56.1% received second-line therapy (Table 1).
Demographic and clinical characteristics and treatment response.
The duration of median follow-up was 19.3 months. The ORR (CR + PR) was achieved in 77.2% of patients, while SD was noted in 7.6% of patients (Table 1).
OS and PFS dataIn the overall study population, 1-year OS and PFS rates were 95.0% and 93.5%, respectively, and median OS and PFS times were 66.3 (95% CI 19.9–112.6) months and 27.4 (95% CI 15.2–39.5) months, respectively.
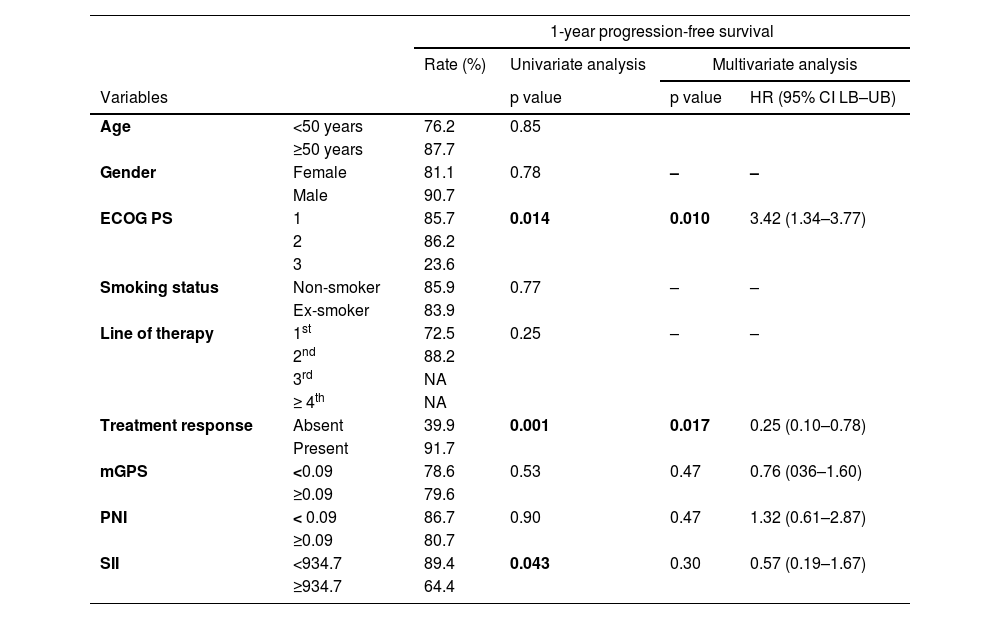
PFS according to clinicopathological variables and inflammation-based prognostic factorsECOG PS category 1 (85.7%) and category 2 (86.2%) were associated with significantly higher 1-year PFS rates than the ECOG PS category 3 (23.6%, p=0.014), while in multivariate analysis, poorer ECOG PS category was a significant determinant of poorer 1-year PFS (HR 3.42, 95% CI 1.34–3.77, p=0.010) (Table 2).
Progression-free survival in relation to clinicopathological and prognostic factors.
HR: Hazards ratio, CI: confidence interval, NA: not applicable, ECOG PS: Eastern Cooperative Oncology Group Performance Status: mGPS: modified Glasgow prognostic score, PNI: Prognostic nutritional index, SII: Systemic immune-inflammation index.
Presence vs. absence of treatment response to crizotinib was associated with significantly higher rates of 1-year PFS (91.7 vs. 39.9%, p=0.001), while in multivariate analysis, the presence of crizotinib treatment response was a significant determinant of higher 1-year PFS (HR 0.25, 95% CI 0.10–0.78, p=0.017) (Table 2).
No significant difference was noted in 1-year PFS rates according to gender, age, smoking status, or the line of therapy (Table 2).
While the 1-year PFS rate was significantly higher (89.4 vs. 64.4%, p=0.043) among patients with low (<934.7) vs. high (≥934.7) SII scores, this finding was not confirmed in the multivariate analysis. No significant difference was noted in 1-year PFS rates according to mGPS and PNI scores (Table 2).
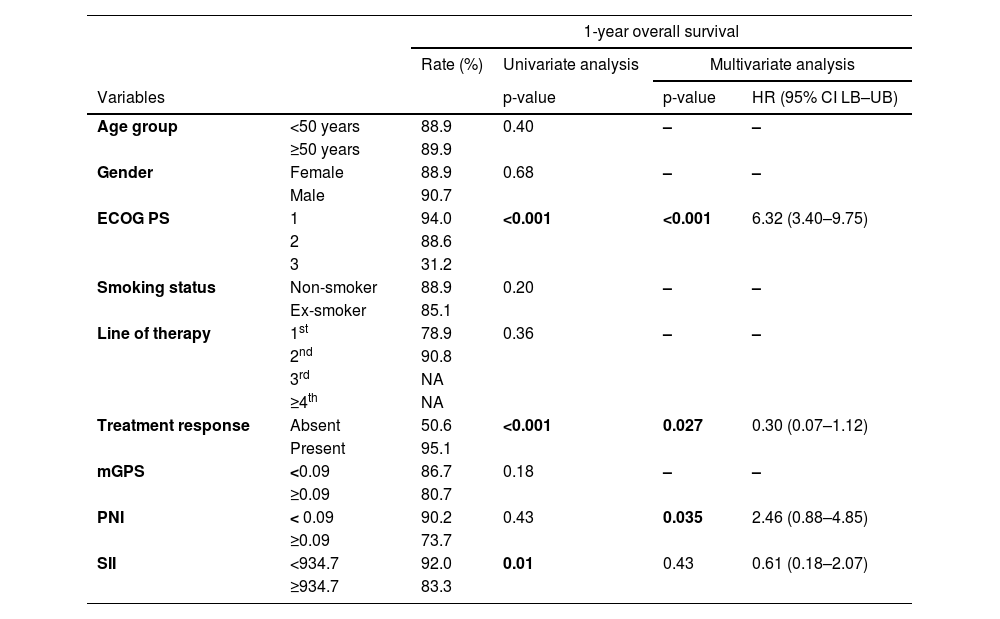
OS according to clinicopathological variables and inflammation-based prognostic factorsECOG PS category 1 (94.0%) and category 2 (88.6%) were associated with significantly higher 1-year OS rates than the ECOG PS category 3 (31.2%, p=0.001), while in multivariate analysis, poorer ECOG PS category was a significant determinant of poorer 1-year PFS (HR 6.32, 95%CI 3.40–9.75, p<0.001) (Table 3).
Overall survival in relation to clinicopathological and prognostic factors.
HR: Hazards ratio, CI: confidence interval, NA: not applicable, ECOG PS: Eastern Cooperative Oncology Group Performance Status; mGPS: modified Glasgow prognostic score, PNI: Prognostic nutritional index, SII: Systemic immune-inflammation index.
Presence vs. absence of treatment response to crizotinib was associated with significantly higher rates of 1-year OS (95.1 vs. 50.6%, p<0.001), while in multivariate analysis, the presence of crizotinib treatment response was a significant determinant of higher 1-year OS (HR 0.30, 95% CI 0.07–1.12, p=0.027) (Table 3).
No significant difference was noted in 1-year OS rates according to gender, age, smoking status or the line of therapy (Table 3).
While the 1-year OS rate was significantly higher (92.0 vs. 83.3%, p=0.01) among patients with low (<934.7) vs. high (≥934.7) SII scores, this finding was not confirmed in the multivariate analysis. Multivariate analysis revealed the PNI values ≥0.09 to be a significant determinant of poorer 1-year OS (HR 2.46, 95% CI 0.88–4.85, p=0.035). No significant difference was noted in 1-year PFS rates according to mGPS scores (Table 3).
Logistic regression analysis for the factors predicting response to crizotinibLogistic regression analysis for the factors predicting the treatment response revealed that higher mGPS scores (OR: 0.1, 95% CI 0.16–1.04; p=0.009) and higher PNI scores (OR: 0.16, 95% CI 0.02–0.55; p=0.035) were associated with poorer ORR (Table 4).
Logistic regression analysis of the predictive factors for the response to crizotinib.
| OR | 95% CI | p-value | |
|---|---|---|---|
| Variables | |||
| ECOG PS | 0.54 | 0.16–4.93 | 0.59 |
| Line of crizotinib therapy | 1.53 | 0.56–4.12 | 0.39 |
| mGPS | 0.10 | 0.16–1.04 | 0.009 |
| PNI | 0.16 | 0.02–0.55 | 0.035 |
| SII | 0.45 | 0.19–4.65 | 0.99 |
OR: Odds ratio, CI: confidence interval, ECOG PS: Eastern Cooperative Oncology Group Performance Status, mGPS: modified Glasgow prognostic score, PNI: Prognostic nutritional index, SII: Systemic immune-inflammation index.
ECOG PS, the line of crizotinib therapy and SII scores had no significant impact on treatment response (Table 4).
DiscussionOur findings in a retrospective cohort of crizotinib-treated ALK-positive metastatic non-resectable NSCLC patients revealed 1-year OS and PFS rates of 95.0% and 93.5%, respectively, along with an ORR of 77.2% after a median 19.3 months of follow-up. Poorer ECOG PS category and absence of crizotinib treatment response were significant determinants of poorer 1-year PFS and OS rates, while higher pre-treatment PNI values (≥0.09) served as a significant predictor of poorer 1-year OS rate and albeit not confirmed in the multivariate analysis, higher pre-treatment SII scores (≥934.7) were associated with poorer 1-year PFS and OS rates in the univariate analysis. Increase in pre-treatment mGPS and PNI scores were also associated with inferior ORR.
Given the predominance of stage III-IV disease and prior lines of crizotinib in our study population comprising patients with ALK-positive metastatic non-resectable NSCLC, our data on survival outcome and ORR appear consistent with data from a previous study on crizotinib-treated patients with ALK-positive advanced NSCLC (n=47), which revealed ORR of 61.7%, disease control rate (DCR) of 93.6%, and PFS of 19 months overall.37 Notably, the authors also indicated a better ORR (63.9% vs. 45.5%), DCR (94.5% vs. 91%), and PFS (19 vs. 11 months) in patients with more than three metastatic sites than those with less sites and better ORR in younger vs. older (>60 years) patients (40 vs. 71.9%) and in the first vs. second/final application of crizotinib (78.2 vs. 45.8%).37
Our findings indicate the potential role of inflammation-based prognostic scores in predicting either the survival outcome (for PNI and SII) or the crizotinib treatment response (for mGPS and PNI) in patients with ALK-positive metastatic NSCLC. This is important given these inflammation-based prognostic scores are considered to be readily available, simple and inexpensive tools, implicating their potential to be routinely considered before treatment for patients with NSCLC.34
Albeit not confirmed in the multivariate analysis, association of pre-treatment elevated SII scores with poorer PFS and OS in the univariate analysis in the current study seems to be consistent with the notion that systemic immune and inflammatory cells, such as neutrophils, monocytes, platelets and lymphocytes, are associated with prognostic value in several malignancies.34,38-40 Notably, association of low SII with less aggressive disease phenotype (with female gender, never smoking status, adenocarcinoma histology, lower pathological TNM stage and low CRP) has also been noted in patients with NSCLC.34
Undoubtedly, given the suggested role of non-steroidal anti-inflammatory drugs in the prevention and treatment of cancer,41 patients with NSCLC who have a high SII are likely to benefit from targeted anti-inflammatory agents with aspirin and non-steroidal anti-inflammatory drugs.34
In the current study, SII was associated with poorer OS and PFS only in the univariate analysis, whereas SII was determined to significantly predict poorer OS in patients with non-resectable ALK-positive NSCLC. However, in a past study on 341 patients who underwent complete resection for NSCLC, SII was noted among the independent factors in predicting overall postoperative cancer-specific survival.34 In another study including 332 patients with newly diagnosed stage III NSCLC, SII ≥660 and PNI ≤52.95 were reported to be significantly associated with worse OS in the univariate analysis, while SII (HR, 2.105; 95% CI 1.481–2.741) and ECOG PS (HR, 1.744; 95% CI 1.158–2.626) were independently correlated with OS in the multivariate analysis.33 Authors indicated SII to be an independent prognostic indicator of poor outcomes in patients with stage III NSCLC and to be superior to other inflammation-based factors in terms of prognostic ability.33
In a previous study on patients with NSCLC who underwent potentially curative resection, authors reported the association of GPS, PNI and SII with overall cancer-specific survival in the univariate analysis, whereas in multivariate analysis GPS was the only inflammation-based prognostic score independently associated with overall cancer-specific survival.27
CRP is a non-specific inflammatory acute-phase protein, and an elevated serum CRP level has been reported to be associated with unfavorable prognosis in patients with NSCLC.34,42 However, in our study, while mGPS was the only inflammation-based score associated with CPR, no significant impact of mGPS was noted on either PFS or OS rates. Furthermore, sub-group analysis of patients with high SII vs. high mGPS revealed longer OS and PFS times in the latter group.
Nutritional status of patients, as commonly reflected by serum albumin levels, is also considered to predict survival in patients with NSCLC.34,43 Among the inflammation-based scores used in this study, mGPS and PNI were defined to be based on albumin and other factors. Thus, given the association of elevated pre-treatment scores for PNI with significantly poorer OS rate in the multivariate analysis as well as predictive role of both mGPS and PNI in the treatment response, our findings seem to indicate a stronger prognostic power of albumin over that of CRP and the possibility that prognostic power of CRP might be off-set in multivariate analysis.
Nonetheless, it should be noted that in a previous study on patients with NSCLC who underwent potentially curative resection, based on superiority of GPS over albumin-based index scores such as ALI and PNI in multivariate analysis, the higher prognostic power of CRP has been emphasized along with the likelihood of the prognostic power of albumin to be off-set in multivariate analysis.34
However, the biological mechanism underlying the association between CRP, albumin, and increased risk of cancer development is unclear. In this context, it is first hypothesized that CRP increase and albumin decrease are probably regulated by proinflammatory cytokines, especially interleukin (IL)-6 and IL-1. These cytokines are also crucial for neoangiogenesis and disease progression, and thus are significantly associated with the increasing risk of lung cancer development.44,45 This interaction between CRP, albumin, and cancer biology may explain the different results between inflammatory scores using CRP and albumin values and other scores using blood cells.
Accordingly, our findings emphasize the likelihood of diversity in the prognostic ability of inflammation-based prognostic factors in patients with NSCLC depending on the presence of targetable oncogenic driver alterations such as rearrangement of ALK gene and the related aggressive clinicopathological course. In this regard, among patients with ALK-positive metastatic or non-resectable NSCLC, PNI rather than SII and GPS seems to be a stronger prognostic indicator of survival outcome, while both GPS and PNI have a significant impact on response to crizotinib treatment.
Certain limitations to this study should be considered. First, owing to the retrospective design establishing the temporality between cause and effect is not possible. Second, given that ALK-positive patients represent a rare subpopulation among patients with NSCLC, the relatively small sample size limits the generalizability of our findings.
Third, lack of longitudinal analysis of the examined inflammation-based markers according to crizotinib cycles and/or disease progression. Forth, no available trAEs-related data and the lack of correlational analyses between inflammatory-based scores and the occurrence of trAEs.
Nevertheless, despite these limitations, given the restricted amount of data available on this subject area, our findings represent a valuable contribution to the literature regarding the potential prognostic markers in crizotinib-treated ALK-positive NSCLC patients.
ConclusionOur findings in a retrospective cohort of crizotinib-treated ALK-positive NSCLC patients indicated the association of elevated pre-treatment SII scores with poorer OS and PFS in univariate analysis. In multivariate analysis, elevated PNI scores were associated with poorer OS, while elevated mGPS and PNI scores were associated with lower treatment response rates. To the best of our knowledge, this is the first report to demonstrate the prognostic significance of PNI and SII in terms of survival outcome and the impact of GPS and PNI on treatment response in crizotinib-treated ALK-positive metastatic or non-resectable NSCLC patients. Accordingly, our findings emphasize potential utility of pre-treatment inflammation-based prognostic scores, in particular albumin-based scores, in predicting survival outcome and treatment response in patients with ALK-positive NSCLC.
FundingNone.
CRediT author statementOmer Fatih Olmez: Conceptualization, Methodology, Writing - Original Draft, Ahmet Bilici: Methodology, Formal analysis, Writing - Review & Editing, Pınar Gursoy: Investigation, Resources, Erdem Cubukcu: Investigation, Resources Abdullah Sakin: Investigation, Resources Taner Korkmaz: Investigation, Resources Ibrahim Cil: Investigation, Resources Burcu Cakar: Investigation, Resources Serkan Menekse: Investigation, Resources Tarik Demir: Investigation, ResourcesOzgur Acikgoz: Validation, Visualization, Jamshid Hamdard: Investigation, Resources, Data Curation.