Current knowledge regarding the measurement properties of the 6-minute walk test (6MWT) in patients with asthma is limited. Therefore, the aim of this study was to assess the test-retest reliability, measurement error and construct validity of the 6MWT and identify determinants of 6-minute walk distance (6MWD) in patients with asthma.
Patients and methods201 asthma patients referred for pre-pulmonary rehabilitation assessment, were retrospectively analyzed (age 61±12 years, 42% male, FEV1 78±27% predicted). Patients performed two 6MWTs on subsequent days using a 30 m straight walking course. Other measurements included resting dyspnea, maximal exercise capacity, body composition, pulmonary function, pulmonary and quadriceps muscle strength and symptoms of anxiety and depression. Measurement error (absolute reliability) was tested using standard error of measurement (SEM), minimal detectable change at 95% confidence interval (MDC95%) and Bland and Altman 95% limits of agreement, whereas test-retest reliability (relative reliability) and construct validity were assessed using the intra-class correlation coefficient (ICC2,1) and correlations, respectively.
ResultsThe 6MWD showed excellent test-retest reliability (ICC2,1: 0.91). The mean change in 6MWD after the second 6MWT was 18m (95%CI 11–24m), with 73% of the patients walking further in the second test. The SEM and MDC95% for the 6MWT were 35 m and 98 m, respectively. The best 6MWD correlated strongly with peak oxygen uptake during CPET and resting dyspnea (r = 0.61–0.64) and had no-to-moderate correlations with body composition, pulmonary function, respiratory and quadriceps muscle strength and symptoms of anxiety and depression (r = 0.02-0.45). Multiple linear regression was able to identify maximal workload, BMI, rollator use, maximal expiratory pressure, FEV1 and DLCO as independent determinants of the best 6MWD (R2 = 0.58).
ConclusionsThe 6MWT was considered to be reliable and valid in patients with asthma, which strengthens its clinical utility. However, the majority of patients demonstrated a considerable learning effect in the second 6MWT, providing a strong rationale for performing two 6MWTs.
Asthma is a chronic respiratory disease with variable expiratory airflow limitation, usually characterized by chronic airway inflammation, that affects many individuals worldwide and inflicts a great burden on society.1 Due to its complex and heterogeneous nature, asthma typically presents with a variety of respiratory and non-respiratory symptoms2,3 and is associated with significant comorbidity and an increased mortality risk.4,5 Currently, asthma management is primarily focused on respiratory symptoms (i.e. pharmacotherapy),6 potentially overlooking the impact of extra-pulmonary manifestations, including exercise limitation and decreased physical activity, which are commonly reported irrespective of impairments in pulmonary function.7–9 Considering the fact that impaired physical functioning is a common feature in patients with chronic respiratory disease,10,11 it is essential for healthcare professionals and clinical scientists to use valid and reliable measures when assessing functional exercise capacity. Tests with good measurement properties are needed to ensure patient progress is well-monitored and above all, accurate. Moreover, daily practice requires tests that are quick and easy to perform in order to guard time management and to retest patients and monitor changes efficiently.
The 6-minute walk test (6MWT) is an easy-to-administer, generally well tolerated and safe field walking test, commonly used as a clinical indicator of functional exercise capacity and a predictor of morbidity and mortality across a wide range of cardiopulmonary diseases.12,13 The reliability and construct validity of the 6MWT have been demonstrated in several patient populations, such as chronic obstructive pulmonary disease (COPD),14 chronic heart failure 15 and idiopathic pulmonary fibrosis.16 Despite the fact that the 6MWT can provide insights in functional limitation in chronic respiratory disease, current knowledge regarding its measurement properties (e.g. reliability, construct validity) in patients with asthma is limited. A considerable learning effect of the 6MWT has been previously established in patients with COPD,13,14 with the majority of individuals showing an increase in 6-minute walk distance (6MWD) when a second test is performed without any intervention or alteration in clinical characteristics. Information regarding this learning effect in patients with asthma is highly limited.17 It seems reasonable to hypothesize that the test-retest reliability of the 6MWT will be good in patients with asthma, since a systematic review by Singh et al. examining field walking tests in chronic respiratory disease stated that intra-class correlation coefficients (ICC's) ranged from 0.82 to 0.99.13 However, the limits of agreement reported in the included studies were large, indicating that there is a certain amount of measurement error and the results from the first test may not be able to predict the outcome of the second test. Therefore, the current study aimed (1) to investigate the measurement error and test-retest reliability of the 6MWT, quantifying a potential learning effect between tests; (2) to evaluate the construct validity of the 6MWT; and (3) to identify the clinical determinants of the best 6MWD in a large cohort of patients with asthma.
MethodsIn a retrospective observational study, 217 adult patients with asthma referred for a pre-pulmonary rehabilitation assessment at Ciro (Horn, the Netherlands) between September 2015 and January 2019, were screened for eligibility. Patients were included in the current analysis if they met the following criteria: respiratory physician-based diagnosis of asthma, based on an initial identification of both a characteristic pattern of symptoms and variable expiratory airflow limitation according to national and international guidelines,1 clinical stability at the time of the assessment (absence of current asthma attack); and two 6MWTs performed on subsequent days. The medical ethics committee of Maastricht University stated that the Medical Research Involving Human Subjects Act (WMO) did not apply for this study and that an official approval of this study by the committee was not required (METC azM/UM 2019-1081).
6MWTTwo 6MWTs were performed according to the official European Respiratory Society (ERS)/American Thoracic Society (ATS) guidelines12,18 on subsequent days using a 30 m straight walking course using cones as turnaround points. Use of walking aids (cane, rollator, etc.) was allowed during the 6MWTs and patients were instructed to take all usual medications. Patients were instructed to walk as far as possible and the distance walked (6MWD) was registered after each test. All tests were supervised by a trained and qualified technician who walked behind the patient, providing them with standardized phrases for encouragement every minute and informing them about the remaining time of the test. Patients were permitted to stop (if required) during the test, but were instructed to resume walking once they were able to. Heart rate (beats/min) and oxygen saturation (SpO2) as measured by pulse oximetry were assessed before, during and at the end of the 6MWTs and perceived dyspnea and leg fatigue (modified Borg scale; range 0-10) were assessed at the start (at rest) and at the end (at peak exertion) of the test. Oxygen supplementation was used if required, and oxygen desaturation during the 6MWT was defined as a drop of ≥4% in SpO2 and an end-SpO2 of <88%. The predicted 6MWD reference values of Troosters et al. were used.19
Other measurementsDemographics, anthropometrics, medication use (including maintenance oral corticosteroids (OCS)), smoking status, asthma attack/hospitalization frequency and the use of oxygen and/or walking aids (rollator or cane) were assessed, as part of standard care. To characterize patients with eosinophilic asthma, blood eosinophils were measured as part of the complete blood count plus differential, using cut-off values of both ≥150 cells/μL and ≥300 cells/μL.20 The modified Medical Research Council (mMRC) scale was used to evaluate the level of functional limitation due to dyspnea in activities of daily living. The mMRC is a five-point scale, ranging from 0 (dyspnea only with strenuous exercise) to 4 (too dyspneic to leave the house or breathless when getting dressed), in which a higher grade indicates higher functional disability due to dyspnea.21
Pulmonary function (Masterlab®, Jaeger, Würzburg, Germany) was measured according to ATS/ERS guidelines using spirometry, with forced expiratory volume in the first second (FEV1) and FEV1/forced vital capacity (FVC) ratio as primary outcomes,22 and whole-body plethysmography, providing residual volume (RV), total lung capacity (TLC) and the RV/TLC ratio.23 Furthermore, respiratory muscle strength, using maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) measurements, was determined.24
Whole-body dual energy X-ray absorptiometry (DEXA) provided information about body composition, by means of lower-limb lean muscle mass (LL-LMM) and calculating fat-free mass (FFM) based on the sum of both lean and mineral bone mass.25 Subsequently, body mass index (BMI) an FFM index (FFMI) was calculated as body weight (kg) and FFM (kg) divided by height squared (m2), respectively. Mood status was assessed with the 14-item Hospital Anxiety and Depression Scale (HADS), in which higher scores are equivalent to more symptoms of anxiety or depression.26 Maximal exercise capacity was evaluated with a symptom limited incremental (+10 W/min) cardiopulmonary exercise test (CPET) performed on a cycle ergometer (Carefusion, Houten, The Netherlands; Oxycon β, Jaeger, Würzburg, Germany), of which maximal power output (Wmax) and peak oxygen consumption (VO2peak in mL/min/kg) were the main outcomes.27,28 Isokinetic quadriceps muscle strength, defined as the highest peak torque in Nm (PTquadriceps), was determined using a computerized dynamometer (Biodex System 4 Pro, Biodex Medical Systems, New York, USA).29,30
Statistical analysesAll statistical analyses were performed using IBM SPSS Statistics 25.0 (SPSS Inc., Chicago, USA) and GraphPad Prism 9.0 (GraphPad Software Inc., California, USA). Descriptive statistics are used to present the data, as appropriate. The data was screened for extreme outliers, after which none were identified. Continuous variables were tested for normality using the Shapiro-Wilk test. The measurement properties of the 6MWT have been assessed according to the taxonomy developed by the COSMIN initiative.31 Accordingly, measurement error (as a measure of absolute reliability) was analyzed using standard error of measurement (SEM; SD∗(√1−ICC)), the minimal detectable change at 95% confidence interval (MDC95%; 1.96*SEM*√2) and Bland and Altman 95% limits of agreement.31 The two-way random ICC with single measures (ICC2,1)32 was calculated to assess the test-retest reliability (as a measure of relative reliability) of the 6MWT.31 Furthermore, the reproducibility of the 6MWT was studied after stratification for sex, age and smoking status. Logistic regression models adjusted for age, sex and BMI were used to identify predictive factors of a change in 6MWD of at least 27 m, which is the minimal clinically important difference (MCID) in patients with asthma,33 between the two 6MWTs.
Construct validity was verified according to the COSMIN guidelines by testing convergent, discriminant and known-groups validity.31 Convergent validity concerns the degree to which a measure is related to other measures of similar constructs, while discriminant validity considers the degree to which a measure can be differentiated from measures of conceptually distinct constructs.34 To investigate convergent and discriminant validity, Pearson or Spearman correlation coefficients were calculated. It was hypothesized that the best 6MWD would show moderate to strong (positive) correlations (r = 0.40–0.79)35 with measures of maximal exercise capacity, as measured with CPET (i.e., Wmax and VO2peak; absolute values), which would support convergent validity. Regarding discriminant validity, we expected only weak to moderate associations (r = 0.20–0.59)35 with absolute values of pulmonary function, body composition, PTquadriceps and symptoms of anxiety and depression (HADS-A and HADS-D). The ability of the 6MWT to discriminate between clinically diverse groups (known-groups validity) was assessed with analyses of variance (one-way ANOVA) with Tukey-HSD post hoc in patients grouped by mMRC score. It was hypothesized that patients with higher mMRC scores would have a lower mean 6MWD. Finally, a stepwise multiple linear regression model was built to identify independent determinants of the best 6MWD. Only explanatory variables with a significant correlation coefficient (p < 0.05) were used in the model (in absolute values). In case of singularity or multicollinearity (i.e., r ≥ 0.70) between two independent variables, only the variable with the strongest association with the best 6MWD was kept in the model. A priori, the level of significance was set at p < 0.05.
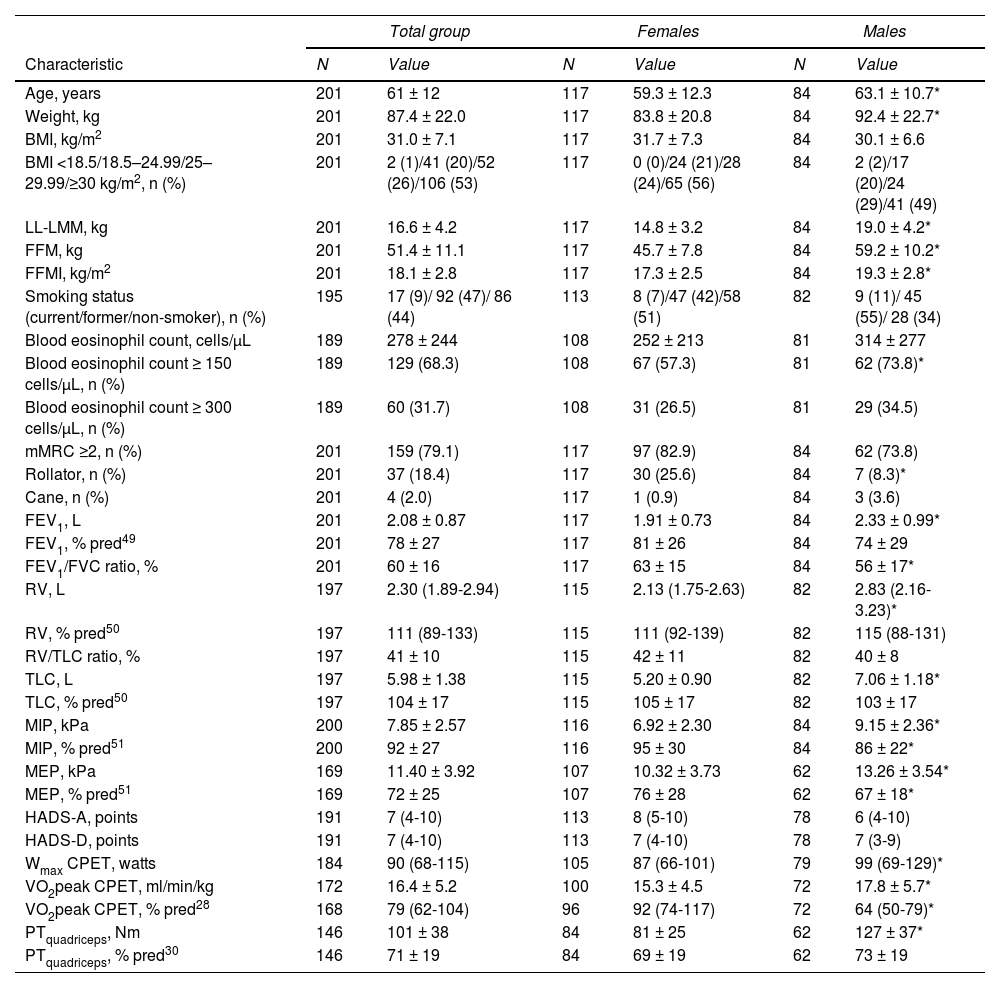
ResultsCharacteristicsTwo patients were not able to perform a 6MWT and 14 patients only performed one 6MWT (due to medical or administrative reasons) and were excluded accordingly. Demographic and clinical characteristics of 201 included patients with asthma (42% male; mean age 61±12 years) are summarized in Table 1. The vast majority of patients (80%) showed abnormal BMI values, with more than half of the sample being obese (BMI ≥30 kg/m2). The proportion of patients on maintenance OCS was 19% (Supplemental Data, Table 1). Based on a blood eosinophil count ≥300 cells/μL, 32% of the patients could be characterized as having eosinophilic asthma. Mean FEV1 was 78±27 % predicted and 79% of the patients had a mMRC dyspnea severity score of 2 or higher. Eighteen percent of the patients used a rollator and 2% used a cane as walking aid. Rollator use was significantly higher in females than in males (p < 0.05; Table 1). Forty-four percent of the patients never smoked, whereas 9% was a current smoker.
Patient characteristics.
| Total group | Females | Males | ||||
|---|---|---|---|---|---|---|
| Characteristic | N | Value | N | Value | N | Value |
| Age, years | 201 | 61 ± 12 | 117 | 59.3 ± 12.3 | 84 | 63.1 ± 10.7* |
| Weight, kg | 201 | 87.4 ± 22.0 | 117 | 83.8 ± 20.8 | 84 | 92.4 ± 22.7* |
| BMI, kg/m2 | 201 | 31.0 ± 7.1 | 117 | 31.7 ± 7.3 | 84 | 30.1 ± 6.6 |
| BMI <18.5/18.5–24.99/25–29.99/≥30 kg/m2, n (%) | 201 | 2 (1)/41 (20)/52 (26)/106 (53) | 117 | 0 (0)/24 (21)/28 (24)/65 (56) | 84 | 2 (2)/17 (20)/24 (29)/41 (49) |
| LL-LMM, kg | 201 | 16.6 ± 4.2 | 117 | 14.8 ± 3.2 | 84 | 19.0 ± 4.2* |
| FFM, kg | 201 | 51.4 ± 11.1 | 117 | 45.7 ± 7.8 | 84 | 59.2 ± 10.2* |
| FFMI, kg/m2 | 201 | 18.1 ± 2.8 | 117 | 17.3 ± 2.5 | 84 | 19.3 ± 2.8* |
| Smoking status (current/former/non-smoker), n (%) | 195 | 17 (9)/ 92 (47)/ 86 (44) | 113 | 8 (7)/47 (42)/58 (51) | 82 | 9 (11)/ 45 (55)/ 28 (34) |
| Blood eosinophil count, cells/μL | 189 | 278 ± 244 | 108 | 252 ± 213 | 81 | 314 ± 277 |
| Blood eosinophil count ≥ 150 cells/μL, n (%) | 189 | 129 (68.3) | 108 | 67 (57.3) | 81 | 62 (73.8)* |
| Blood eosinophil count ≥ 300 cells/μL, n (%) | 189 | 60 (31.7) | 108 | 31 (26.5) | 81 | 29 (34.5) |
| mMRC ≥2, n (%) | 201 | 159 (79.1) | 117 | 97 (82.9) | 84 | 62 (73.8) |
| Rollator, n (%) | 201 | 37 (18.4) | 117 | 30 (25.6) | 84 | 7 (8.3)* |
| Cane, n (%) | 201 | 4 (2.0) | 117 | 1 (0.9) | 84 | 3 (3.6) |
| FEV1, L | 201 | 2.08 ± 0.87 | 117 | 1.91 ± 0.73 | 84 | 2.33 ± 0.99* |
| FEV1, % pred49 | 201 | 78 ± 27 | 117 | 81 ± 26 | 84 | 74 ± 29 |
| FEV1/FVC ratio, % | 201 | 60 ± 16 | 117 | 63 ± 15 | 84 | 56 ± 17* |
| RV, L | 197 | 2.30 (1.89-2.94) | 115 | 2.13 (1.75-2.63) | 82 | 2.83 (2.16-3.23)* |
| RV, % pred50 | 197 | 111 (89-133) | 115 | 111 (92-139) | 82 | 115 (88-131) |
| RV/TLC ratio, % | 197 | 41 ± 10 | 115 | 42 ± 11 | 82 | 40 ± 8 |
| TLC, L | 197 | 5.98 ± 1.38 | 115 | 5.20 ± 0.90 | 82 | 7.06 ± 1.18* |
| TLC, % pred50 | 197 | 104 ± 17 | 115 | 105 ± 17 | 82 | 103 ± 17 |
| MIP, kPa | 200 | 7.85 ± 2.57 | 116 | 6.92 ± 2.30 | 84 | 9.15 ± 2.36* |
| MIP, % pred51 | 200 | 92 ± 27 | 116 | 95 ± 30 | 84 | 86 ± 22* |
| MEP, kPa | 169 | 11.40 ± 3.92 | 107 | 10.32 ± 3.73 | 62 | 13.26 ± 3.54* |
| MEP, % pred51 | 169 | 72 ± 25 | 107 | 76 ± 28 | 62 | 67 ± 18* |
| HADS-A, points | 191 | 7 (4-10) | 113 | 8 (5-10) | 78 | 6 (4-10) |
| HADS-D, points | 191 | 7 (4-10) | 113 | 7 (4-10) | 78 | 7 (3-9) |
| Wmax CPET, watts | 184 | 90 (68-115) | 105 | 87 (66-101) | 79 | 99 (69-129)* |
| VO2peak CPET, ml/min/kg | 172 | 16.4 ± 5.2 | 100 | 15.3 ± 4.5 | 72 | 17.8 ± 5.7* |
| VO2peak CPET, % pred28 | 168 | 79 (62-104) | 96 | 92 (74-117) | 72 | 64 (50-79)* |
| PTquadriceps, Nm | 146 | 101 ± 38 | 84 | 81 ± 25 | 62 | 127 ± 37* |
| PTquadriceps, % pred30 | 146 | 71 ± 19 | 84 | 69 ± 19 | 62 | 73 ± 19 |
Summary variables are presented as n (%) for discrete variables, mean ± standard deviation for quantitative variables or median (Interquartile range) for skewed variables. * p<0.05 females vs. males. Abbreviations. BMI: body mass index; LL: lower-limbs; LMM: lean muscle mass; FFM: fat-free mass; FFMI: fat-free mass index; mMRC: modified Medical Research Council; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; RV: residual volume; TLC: total lung capacity; MIP: Maximal inspiratory pressure; MEP: Maximal expiratory pressure; HADS-A: Hospital Anxiety and Depression Scale, Anxiety subscale; HADS-D: Hospital Anxiety and Depression Scale, Depression subscale; Wmax: maximal achieved workload; CPET: cardiopulmonary exercise test; VO2peak: peak oxygen consumption; PTquadriceps: isokinetic peak torque of the quadriceps muscle.
On average, patients walked 392 m (95% CI 376–408 m) in the first 6MWT and 410 m (95% CI 393–427 m) in the second 6MWT. The mean change in 6MWD was 18 m (95% CI 11–24 m) or 5% (p < 0.001). Seventy-three percent of the patients improved their 6MWD in the second test, with 39% having an increase of at least 27 m (MCID of the 6MWT in patients with asthma).33 11% of the patients decreased their 6MWD with ≥27 m. After stratification for sex, age and smoking status, no differences in terms of reproducibility of the 6MWT between these subgroups were shown (Supplemental Data, Table 2).
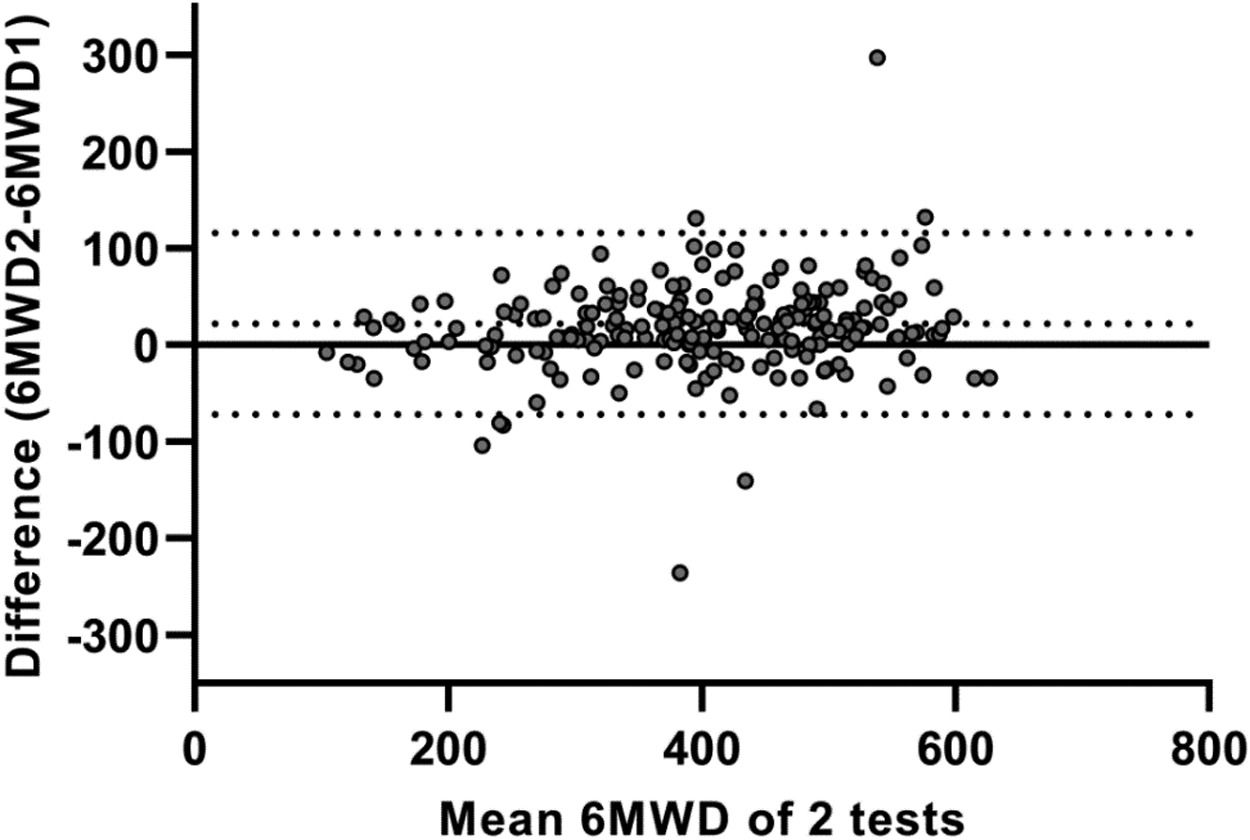
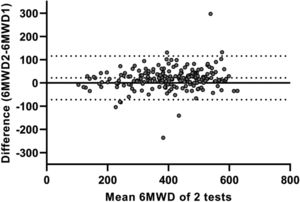
Test-retest reliability between the two 6MWTs was excellent, with an ICC value of 0.91 (95%CI 0.86–0.94; p < 0.001). The SEM and MDC95% for the 6MWT were 35 m and 98 m, respectively. Bland and Altman plots confirmed that the majority of patients increased their 6MWD during the second 6MWT (average difference: 18m; Fig. 1). The limits of agreement between first and second 6MWTs ranged from -75 to 110 m. Logistic regression models showed that none of the specific patient characteristics were associated with a lower or higher likelihood of having a meaningful increase in 6MWD (≥27 m33) in the second 6MWT (Supplemental Data, Table 3).
Bland and Altman plot illustrating the difference in six-minute walking distance (6MWD; in meters) between two 6-minute walk tests (6MWTs) conducted on a 30 m straight point-to-point course, plotted against the mean value of these two tests in patients with asthma. The central continuous line represents the zero line; the central dotted line corresponds to the average difference (bias) between the two 6MWTs (18 m); and the lower and upper dotted lines correspond to the lower (−75 m) and upper (110 m) limits of agreement, respectively.
On average, the change in oxygen saturation (ΔSpO2) during the first and second 6MWT was -1.9% and -2.0% (Supplemental Data, Table 4). Of the 201 patients, 20 patients (10.0%) showed a desaturation in 6MWT1 and 25 patients (12.4%) in 6MWT2. The reliability of ΔSpO2 when two 6MWTs were performed was only modest (ICC 0.68; p < 0.001). When comparing the change in heart rate and Borg symptom scores, relative reliability was modest with ICC's ranging from 0.44–0.57 (p < 0.001, Supplemental Data, Table 4).
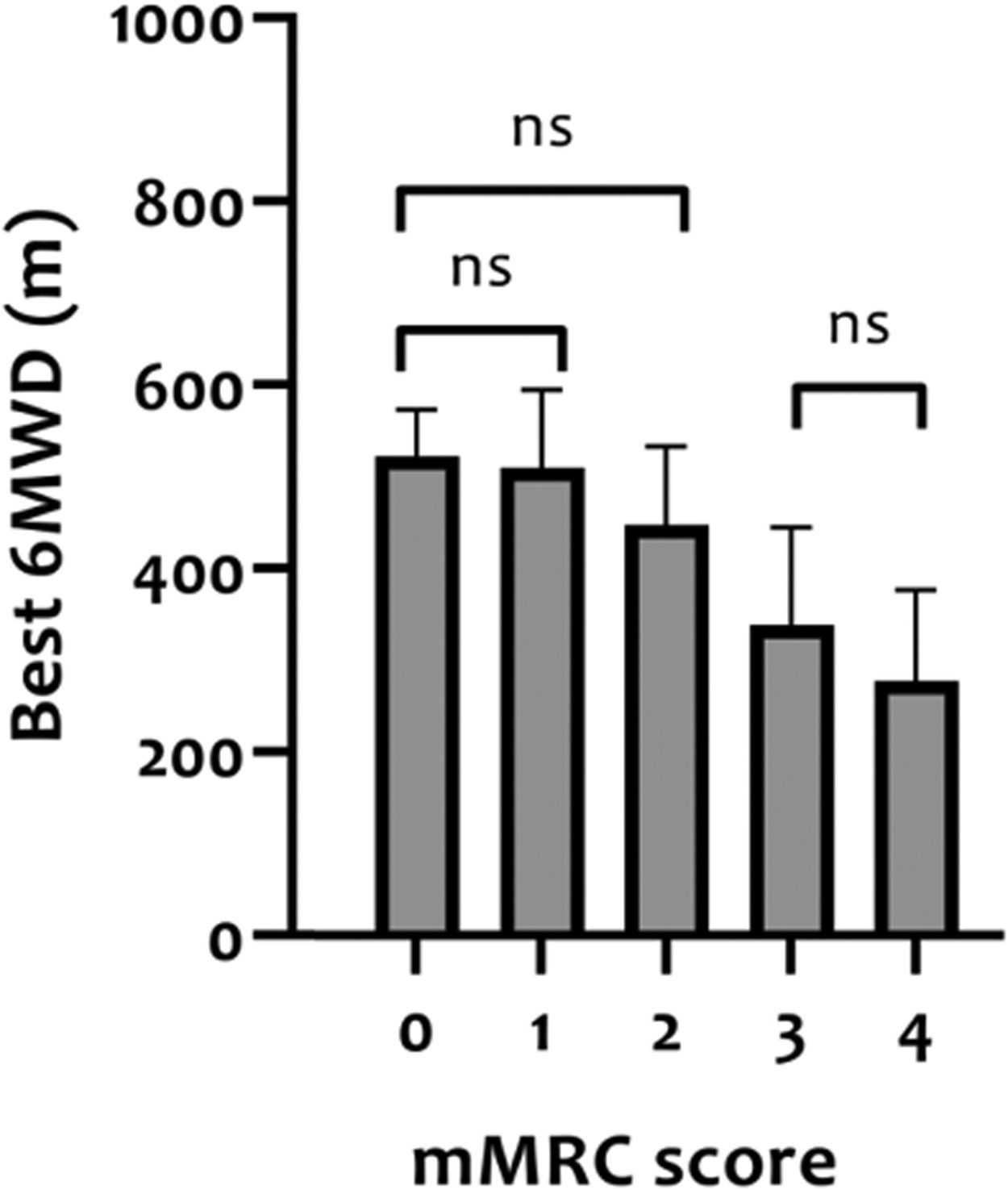
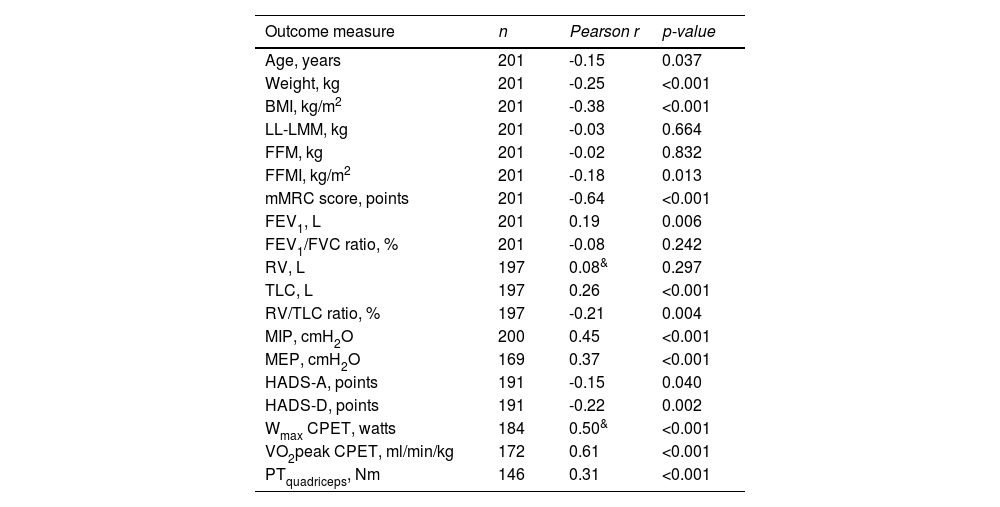
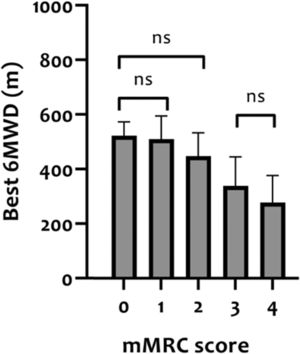
Construct validity of 6MWDThe mean 6MWD on the best test was 418 m (95% CI 402-435 m) or 68% of the predicted values (95% CI 65-70% predicted). In Table 2, correlation coefficients between the best 6MWD and other outcomes are presented. A strong correlation was found with VO2peak (r = 0.61) and mMRC score (r = -0.64), whereas Wmax presented a moderate correlation (r = 0.50). Regarding pulmonary function, FEV1, RV and FEV1/FVC ratio showed very weak correlations (r = 0.08-0.19), and TLC and RV/TLC ratio weak correlations (r = 0.26 and -0.21) with best 6MWD. Very weak-to-moderate correlations were found with age, measures of body composition (BMI, FFM, and LL-LMM), MIP/MEP, PTquadriceps, HADS-A and HADS-D (i.e., r = 0.02–0.45; Table 2). Mean 6MWD decreased as functional impairment attributable to dyspnea (mMRC scores) increased, supporting known-groups validity (Fig. 2). ANOVA showed a significant overall difference (p < 0.001) in best 6MWD among mMRC groups, with non-significant Tukey-HSD post hoc mean differences between mMRC score 0 and 1-2 and between mMRC score 3 and 4 (Fig. 2).
Correlations between best 6-minute walk distance and other outcome measures in patients with asthma.
See Table 1 for definition of abbreviations. & Spearman's ρ correlation coefficient
Distance covered in the 6MWT (best 6MWD) stratified according to modified Medical Research Council (mMRC) resting dyspnea severity scores in patients with asthma. The ability of the 6MWT to discriminate between clinically diverse groups (known-groups validity) was assessed with analyses of variance (one-way ANOVA) with Tukey-HSD post hoc. ns = non-significant difference between groups.
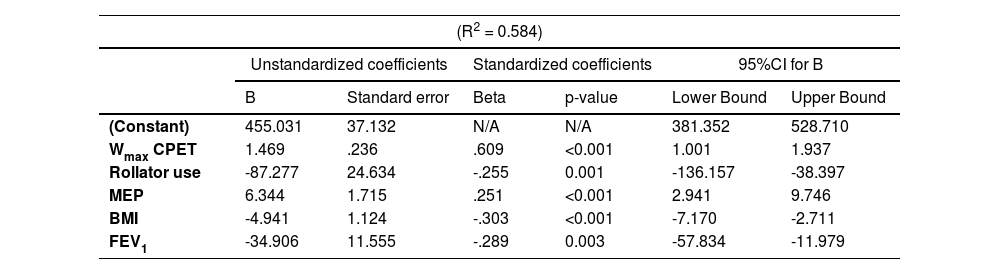
All the variables with a significant correlation coefficient (p < 0.05) were considered in a stepwise multiple linear regression model (Table 3). Weight had to be excluded due to singularity with BMI and VO2peak had to be excluded due to multicollinearity with Wmax (i.e., r > 0.70). As there was a significant difference in best 6MWD between male (463±105 m) versus female patients (386±119 m), sex was included as an independent variable in the model. In addition, the use of a rollator during the 6MWT was added to the regression model, since additional analyses showed that several patient characteristics and 6MWT performance were statistically different between patients who required a rollator and patients who did not (Supplemental Data, Table 5). Subsequently, the following variables were included in the model assessing the best 6MWD: age, sex, rollator use, BMI, FFMI, mMRC, FEV1, RV/TLC ratio, TLC, MIP, MEP, PTquadriceps, HADS-A and HADS-D scores, and Wmax. Out of these, Wmax, rollator use, MEP, BMI and FEV1 were shown to be independent determinants of the best 6MWD, explaining 58.4% of the variability (Table 3).
Stepwise multiple linear regression analysis with the best 6MWD as the dependent variable.
See Table 1 for definition of abbreviations. Variables entered in model: age, sex, rollator use, BMI,
FFMI, mMRC, FEV1, TLC, MIP, MEP, PTquadriceps, HADS-A and HADS-D scores, and Wmax.
The 6MWT showed good test-retest reliability in patients with asthma when two tests were performed on subsequent days. Nevertheless, in the majority of patients the distance walked in the second test improved, indicating a consistent learning effect. As a matter of fact, about four in every ten patients increased their 6MWD in the second test with at least 27 m, which is considered the MCID of the 6MWT in patients with asthma.33 This was supported by Bland and Altman analyses, presenting limits of agreement largely exceeding this 27 m cutoff. Our results also show that there is a large interval (MDC95% of 98 m) within which a patient's true score will lie with repeated testing, 95% of the time, which suggests important within-participant variability on repeated testing in this population. Interestingly, none of the possible determinants were able to predict a clinically important increase in the second 6MWT in this patient population. Consequently, it is recommended to test all patients with asthma twice.
Numerous studies have reported a learning effect when repeated 6MWTs are performed in patients with COPD, with a pooled mean improvement of 26.3 m.13 To date, there is only one study available supporting a learning effect in asthma when two 6MWTs are performed on the same day.17 The learning effect of 19 m (95% CI 11–27 m) or 4%, with 80% of the patients walking further in the second test, is in line with the 18 m (or 5%) reported in our study, in which 73% of the patients improved their 6MWD in the second test. This is consistent with the general assumption that the learning effect is most probably present in different chronic respiratory diseases and even in healthy subjects.13,36–38 The few studies reporting exercise intolerance in patients with asthma using the 6MWT, all state that 6MWTs were performed in accordance with international guidelines, without specifying the use of a second test, hampering the interpretation of these results.3,7,38,39 The learning effect established in the current study indicates that the vast majority of patients could have an underestimation regarding their baseline functional exercise capacity if only one 6MWT was performed. This is supported by the magnitude of the measurement error of the 6MWT in this study, which suggests that there is important within-patient variability on repeated testing. Thus, the well-known learning effect on the second 6MWT13 is demonstrated for the first time in a large sample of asthmatics and our data provide a strong rationale for performing two 6MWTs in clinical settings as well as in clinical studies. To date, it remains unclear whether the learning effect extends beyond a second 6MWT. Current knowledge regarding the potential use of a third 6MWT is based on small studies in patients with COPD, showing inconsistent results.40,41
Strong associations with oxygen uptake during CPET and dyspnea during daily life demonstrate a good convergent validity of the 6MWT in patients with asthma, supporting the general conceptualization of the 6MWT to be a test of functional exercise performance.12 In addition, 6MWD and measures of constructs that theoretically should not be highly related to it (e.g. body composition, pulmonary function, symptoms of anxiety and depression) were, in fact, not found to be highly correlated, strengthening the discriminant validity of the 6MWT in this patient population. For example, FEV1 was very weakly associated with the distance covered in the 6MWT, which is in line with prior studies,7,10 and only increases the added value of a field exercise test, such as the 6MWT. Indeed, based on the lung function attributes a healthcare professional cannot adequately estimate the exercise performance of the individual patient.13 So, a field exercise test needs to be conducted to better understand the exercise capacity of an individual patient with asthma.
A considerable number of independent determinants of 6MWD in patients with asthma were identified, explaining about half of its variance, suggesting that there are other contributors to 6MWD that are not yet completely understood. For instance, dynamic hyperinflation, which has been proven to play a major role in exercise limitation in COPD,42 has also been reported in patients with asthma, with studies showing a direct association with worse overall health, lower wellbeing and impaired activities of daily life.43 Interestingly, severe asthmatic patients seem to develop dynamic hyperinflation during a 6MWT to the same extent as individuals with COPD, without leading to changes in dyspnea perception.44
Methodological strengths and limitationsThe current in-depth analyses of the measurement properties of the 6MWT in patients with asthma are novel and of clinical importance, potentially enhancing the standard operating procedures for the 6MWT in this patient population. Methodological factors that could potentially affect test performance were kept constant when conducting the tests. 6MWTs were conducted by trained and certified personnel using standardized phrases of instruction and encouragement throughout the tests. Additionally, both 6MWTs were performed with the same provision of supplemental oxygen and walking aids used in the first test were also used in the second test.
We acknowledge the fact that the asthma patients included in the current study were referred for pulmonary rehabilitation, resulting in a selected group of patients. Regardless of the fact that our sample showed a broad age range (20-84 years) and a fairly even distribution of males and females, only a minority of patients was aged below 40 years. Of course, this limits the interpretation of the 6MWT measurement properties in patients with asthma to a more narrow age range population. It is worth noting that narrow age-ranges could be identified in most studies conducted in healthy adults to provide reference values for the 6MWT. This also holds true for the reference values19 used in the current study, which are currently the best option available when assessing a Dutch population. Future research is needed in a large sample from the general population, representing all age groups and well balanced in terms of sex, to formulate new reference values for use in daily clinical practice.
Unfortunately, information regarding comorbidities and functional balance, which have been shown to be variables that can influence the improvement of the second 6MWT in patients with COPD,14,45 have not been systematically assessed during the baseline pulmonary rehabilitation assessment. Furthermore, the evaluation of known-groups validity in our study is limited, since asthma severity according to GINA guidelines1 was not determined. Generally, asthma disease severity is assessed retrospectively by a physician after the patient has been on controller treatment for several months.6,46 However, upon first referral to a pulmonary rehabilitation program, patients may often present a suboptimal disease control due to ineffective or inappropriate treatment or asthma that remains uncontrolled despite sufficient treatment,1 making an adequate examination of disease severity difficult. As a result, the present study aimed to classify patients based on the type of drug (OCS) that they were prescribed, without inferring severity, other than the fact that a referral to a tertiary center was made. Lastly, we cannot completely rule out the fact that some patients present with clinical features of both asthma and COPD. Asthma patients entering pulmonary rehabilitation are complex in terms of symptoms, frequency of asthma attacks, oral corticosteroid dependency, current smoking, high prevalence of obesity and other comorbidities,47 which might (at least in part) result in clinical features overlapping with patients with COPD.48
ConclusionThe current study demonstrated excellent test-retest reliability and construct validity of the 6MWT in a large cohort of asthmatics of which the majority was older than 40 years, supporting the use of the 6MWT as a primary end-point in future clinical trials. Nevertheless, our data suggest a considerable learning effect when two tests are conducted. In about 40% of the patients, this learning effect is large enough to be clinically important (≥27 m) when the 6MWT is used to assess functional exercise performance and evaluate patient progress. The exact predictive factors of this improvement in walking distance could not be determined. Therefore, it is highly recommended to perform at least two 6MWTs in each patient, after which the best 6MWD should be reported.
The scientific work of Drs. Roy Meys and Drs. Anouk A.F. Stoffels is financially supported by Lung Foundation Netherlands under Grant number 5.1.18.232.