It has been demonstrated that there are a lot of different prognostic factors which are worthy of consideration whereas diabetes mellitus (DM) has not been clearly or consistently identified as a prognostic value in advanced non-small cell lung cancer (NSCLC). The aim of this study was to investigate the prognostic significance of the characteristics of patients in advanced NSCLC. Specifically, we investigated the impact of DM for progression-free survival (PFS) and overall survival (OS) in patients receiving first-line platinum-based doublets chemotherapy.
MethodsWe retrospectively reviewed 442 patients with advanced NSCLC. DM and other potential prognostic variables were chosen for analysis in this study. Univariate and multivariate analyses were conducted to identify prognostic factors associated with survival.
ResultThe results of univariate analysis for OS were identified as having prognostic significance: performance status (p<0.001), stage (p<0.001), DM (p<0.001), liver metastasis (p=0.02) and brain metastasis (p<0.001). Stage, diabetes mellitus, and liver metastasis were identified as having prognostic significance for PFS.
Multivariate analysis showed that poor performance status, presence of DM and advanced stage were considered independent negative prognostic factors for OS (p 0.001, p<0.001 and p<0.001 respectively). Furthermore, DM and stage were considered independent negative prognostic factors for PFS (p 0.005 and p 0.001 respectively).
ConclusionIn conclusion, DM at the time of diagnosis was associated with the negative prognostic importance for PFS and OS in the advanced stage patients who were receiving first-line platinum-based doublets chemotherapy. In addition poor performance status and advanced stage were identified as negative prognostic factors.
Existem vários factores de prognóstico previamente estabelecidos no carcinoma pulmonar de não pequenas células (NSCLC) avançado. Até à presente data, a diabetes mellitus (DM) não foi identificada de forma consistente como potencial factor de prognóstico neste contexto. O objectivo do presentes estudo é investigar o significado prognóstico das diferentes características dos doentes com NSCLC avançada. Especificamente, investigámos o impacto da DM para a sobrevivência livre de progressão (PFS) e sobrevivência global (OS) em doentes em regime duplo de quimioterapia de primeira linha à base de platina.
MétodosAnalisámos retrospectivamente 442 doentes com NSCLC avançado. Foram escolhidas a DM e outras potenciais variáveis de prognóstico para a análise neste estudo. Foram realizadas análises univariadas e multivariadas para identificar os factores de prognóstico associados à sobrevivência.
ResultadosOs resultados de análise univariada para OS identificaram como tendo significância para o prognóstico: estado geral (p <0,001), estádio (p <0,001), DM (p <0,001), metástases hepáticas (p=0,02) e metástases no cérebro (p <0,001). O estádio, diabetes mellitus e as metástases hepáticas foram identificadas como tendo significância de prognóstico para a PFS.A análise multivariada mostrou que um mau estado geral, a presença de DM e um estádio avançado foram considerados factores de prognóstico negativo independentes para OS (p 0,001; p <0,001 e p <0,001; respectivamente). Além disso, a DM e estádio foram considerados factores de prognóstico negativo independentes para a PFS (p 0,005 e p 0,001; respectivamente).
ConclusõesEm conclusão, a DM na altura do diagnóstico comportou-se como um fator de prognóstico negativo para a PFS e OS nos doentes em estádio avançado que estavam em regime duplo de quimioterapia de primeira linha à base de platina. Além do mau estado geral, os estádios avançados foram identificados como factor de prognóstico negativo.
Lung cancer is the most common of all cancer deaths in both men and women worldwide. NSCLC represents 80–85% of all diagnosed lung cancers cases.1 Surgery is the only curative treatment option for patients with NSCLC. At the time of diagnosis, two-third of patients with lung cancer are diagnosed with locally advanced or metastatic disease. The median survival time for advanced disease is 5.8–12.6 months and the overall 5-year survival rate among this patient population is still less than 10%.2,3 Systemic chemotherapy with the platinum-based doublets are currently recommended as a standard of first-line chemotherapy for treatment of NSCLC.4
An increased incidence of the NSCLC is connected with aging,5 which explains why NSCLC frequently appears simultaneously with other age-related diseases such as diabetes mellitus (DM). Various studies have indicated that among patients with colorectal,6 pancreatic,7 breast,8 or liver9 cancer, existing DM was associated with a lower long-term survival. The prognostic significance of pre-existing DM in patients with lung cancer was evaluated.10–18 Three studies10–12 reported a decrease in survival in patients with DM compared to those without DM. On the other hand, several studies have shown that DM was associated with an equal13–15 or an increased chance of survival.16–18 The effect of DM on patients with NSCLC remains uncertain. In addition to this, DM has not been clearly or consistently identified as a prognostic value for the recurrence or progression of advanced NSCLC.6,11–14 The aim of this study was to investigate the impact of DM for PFS and OS on the patients receiving first-line platinum-based doublets chemotherapy.
MethodsWe retrospectively reviewed 442 patients with histological or cytological proven NSCLC who were receiving first-line platinum-based doublets chemotherapy between August 2002 and February 2012. They had to meet the following inclusion criteria; (1) they had a histological or cytological diagnosis of locally advanced and/or metastatic NSCLC; (2) they were 18 years of age or older; (3) they had had no previous chemotherapy or radiotherapy; (4) they had to have a measurable disease, as defined by Response Evaluation Criteria in Solid Tumors RECIST.
Patients were identified as having DM on the basis of elevated fasting glucose level (>126mg/dL), and a history of DM or medication use, such as insulin or oral hypoglycemic agents. Prevalent hypertension was defined as systolic pressure ≥140mm Hg and/or diastolic ≥90 diastolic and/or currently taking antihypertensive medications.
Twelve potential prognostic variables were chosen on the basis of previously published clinical trials.19–24 The variables were divided into categories: age (<70 or ≥70), gender (male or female), Eastern Cooperative Oncology Group (ECOG) performance status (0–1 or 2–3), stage (III or IV), histology (squamous cell carcinoma or adenocarcinoma), weight loss ≥5% within the previous 3 months (present or absent), DM (present or absent), hypertension (present or absent), smoking history (present or absent), second-line chemotherapy (present or absent), liver metastasis (present or absent) and brain metastasis (present or absent) at the time of first-line chemotherapy administration.
All of the analyses were performed using the SPSS statistical software program package (SPSS version 11.5 for windows). The differences in the clinical characteristics between the two groups were analyzed by a chi-square test and a student t test. OS was calculated from the start of the first cycle of chemotherapy to the date of death from any cause or the date of the last follow-up. PFS was defined as the time from the first day of chemotherapy till progression or death. OS and PFS were estimated using the Kaplan–Meier method. The Cox proportional hazards regression model was used to determine statistical significant variables related to survival. Differences were assumed to be significant when P value was less than 0.05.
ResultsBetween August 2002 and February 2012, 442 patients with advanced NSCLC were enrolled in this study. There were 66 patients (M: 56, F: 10) on DM side and 376 patients (M: 328, F: 48) on the non-diabetic side. DM patients were older than the non-diabetic patients (median 60 versus 58, P>0.05). HT was more common on the DM side than on the non-diabetic one (22.7% versus 8.2%, p<0.0001). There was no statistical difference between patients with the DM and the non-diabetic group in terms of gender, performance status, stage, histology, weight loss, smoking history, first-line chemotherapy, first-line chemotherapy cycles, second-line chemotherapy (p>0.05). The patients’ baseline characteristics are listed in Table 1.
Characteristics of diabetic and non-diabetic NSCLC patients.
| Characteristic | With DM | Without DM | p | ||
| No | (%) | No | (%) | ||
| Enrolled patients. | 66 | 14.9 | 376 | 85.1 | |
| Sex | |||||
| Male | 56 | 84.8 | 328 | 87.2 | P>0.05 |
| Female | 10 | 15.2 | 48 | 12.8 | |
| Age, years | |||||
| <70 | 54 | 81.8 | 327 | 87.0 | P>0.05 |
| ≥70 | 12 | 18.2 | 49 | 13.0 | |
| Performance status (%) | |||||
| 0–1 | 30 | 45.5 | 218 | 58.0 | P>0.05 |
| 2–3 | 16 | 24.2 | 100 | 26.6 | |
| Unknown | 20 | 30.3 | 58 | 15.4 | |
| Smoking history | |||||
| Current or former | 40 | 60.6 | 250 | 66.5 | |
| Never | 6 | 9.1 | 55 | 14.6 | P>0.05 |
| Unknown | 20 | 30.3 | 71 | 18.9 | |
| Weight loss | |||||
| Yes | 5 | 7.6 | 77 | 20.5 | |
| No | 40 | 60.6 | 238 | 63.3 | P>0.05 |
| Unknown | 21 | 31.8 | 61 | 16.2 | |
| Metastatic sites (%) | |||||
| Liver | 10 | 15.1 | 43 | 11.4 | P>0.05 |
| Brain | 11 | 16.7 | 56 | 14.9 | |
| Stage (%) | |||||
| III | 28 | 42.4 | 160 | 42.6 | |
| IV | 38 | 57.6 | 216 | 57.4 | P>0.05 |
| Hypertension | |||||
| Yes | 15 | 22.7 | 31 | 8.2 | |
| No | 51 | 77.3 | 345 | 91.8 | P<0.001 |
| First-line chemotherapy | |||||
| GemCis | 19 | 28.8 | 115 | 30.5 | |
| DocCis | 23 | 34.8 | 132 | 35.0 | P>0.05 |
| PacCis | 19 | 28.8 | 93 | 24.7 | |
| Other | 13 | 7.6 | 37 | 9.8 | |
| Second-line chemotherapy | 19 | 28.6 | 107 | 28.7 | P>0.05 |
The results of univariate analysis for OS are summarized in Table 2. Among the twelve variables of univariate analysis, five variables were identified as having prognostic significance: Performance status (p<0.001), stage (p<0.001), diabetes mellitus (p<0.001), liver metastasis (p=0.02) and brain metastasis (p<0.001).
Univariate analysis of OS by categorical variable.
| Variable | Log-rank test value | Degrees of freedom | p-Value |
| Sex | 0.87 | 1 | 0.35 |
| Age | 1.23 | 1 | 0.26 |
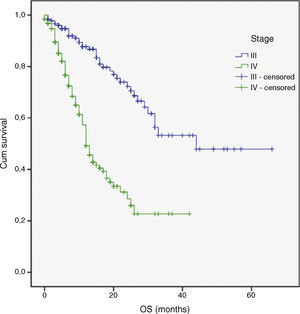
| Stage | 52.5 | 1 | <0.001 |
| Smoking history | 2.12 | 1 | 0.14 |
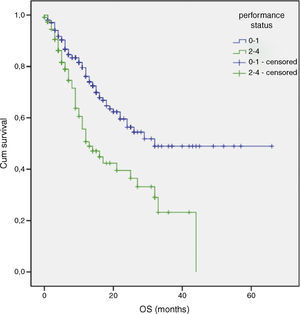
| Performance status | 14.8 | 1 | <0.001 |
| Histology | 0.18 | 1 | 0.66 |
| Weight loss | 0.32 | 1 | 0.85 |
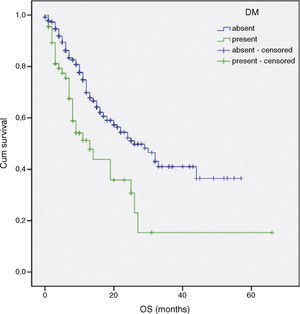
| DM | 15.1 | 1 | <0.001 |
| Hypertension | 2.5 | 1 | 0.10 |
| Second-line chemotherapy | 3.0 | 1 | 0.08 |
| Metastatic sites | |||
| Liver | 5.3 | 1 | 0.02 |
| Brain | 29.4 | 1 | <0.001 |
The results of univariate analysis for PFS are summarized in Table 3. Three variables were identified as having prognostic significance: stage (p<0.001), diabetes mellitus (p<0.001), and liver metastasis (p=0.04).
Univariate analysis of PFS by categorical variable.
| Variable | Log-rank test value | Degrees of freedom | p-Value |
| Sex | 1.65 | 1 | 0.19 |
| Age | 2.47 | 1 | 0.11 |
| Stage | 19.8 | 1 | <0.001 |
| Smoking history | 0.19 | 1 | 0.65 |
| Performance status | 0.005 | 1 | 0.94 |
| Histology | 0.16 | 1 | 0.68 |
| Weight loss | 0.29 | 1 | 0.58 |
| DM | 14.5 | 1 | <0.001 |
| Hypertension | 1.57 | 1 | 0.20 |
| Second-line chemotherapy | 0.62 | 1 | 0.43 |
| Metastatic sites | |||
| Liver | 3.88 | 0.04 | |
| Brain | 2.85 | 1 | 0.09 |
Multivariate analysis included the prognostic significance factors in univariate analysis. The results of multivariate analysis are shown in Table 4. Multivariate analysis by Cox proportional hazard model showed that poor performance status, presence of DM and advanced stage were considered independent negative prognostic factors for OS (p 0.001, p<0.001 and p<0.001 respectively) (Figs. 1–3). Furthermore, DM and stage were considered as independent negative prognostic factors for PFS (p 0.005 and p 0.001 respectively).
In this retrospective study, we set out to clarify the impact of DM on the prognosis of patients with NSCLC. The aim of this study was to investigate the impact of DM for PFS and OS on the patients receiving first-line platinum-based doublets chemotherapy.
The prevalence of DM and cancer is increasing in current society. In our study, the prevalence of DM in NSCLC patients was 14.9%, about twice as high as the global prevalence of diabetes.19 Epidemiological evidence indicates that DM was found to be common in some cancers such as breast, colorectum, liver, endometrium, and pancreas cancer.24–28 The prevalence of DM in newly diagnosed cancer patients is reported to be 8–18% in the United States.29 Diabetes is generally characterized by insulin resistance and hyperinsulinemia.30 It has been claimed that hyper-insulinemia can lead to increases in the level of growth hormones for malignant tumors, which often over-express insulin receptors and insulin-like growth factor.11,31
A number of very different trials have linked pre-existing DM at the time of diagnosis with an increased mortality rate for patients with pancreatic,8 hepatocellular,9 or breast10 cancer. However, there are few studies about the impact of diabetes mellitus for survival in lung cancer.10–18 The evidence is limited and conflicting, although prior studies16–18 have shown that pre-existing DM at the time of diagnosis is strongly associated with an increase in OS. On the other hand, other studies have shown the association between DM and an equal13–15 or an increased survival rate.16–18
The current study demonstrated that patients with DM (n=66) exhibited a significant increase in mortality and recurrence compared with those without DM (n=376). Our retrospective study review suggests that DM is associated with independent risk factors for survival. There may be several reasons for this. First, patients with DM may present with more advanced NSCLC.32 Because of concomitant treatment of the chronic diseases associated with DM, patients may not undergo routine screening for lung cancer.33 Second, pre-existing diabetes may have a greater risk of chemotherapy-related toxicity such as febrile neutropenia, infection.32 Third, patients with DM may receive less aggressive treatment, (including chemotherapy, radiotherapy, and/or surgery).32
A poor PS is usually accepted as a negative prognostic factor for all cancer patients.34,35 The importance of PS was also confirmed in advanced NSCLC patients.21,22 Our results demonstrated that PS is associated with independent negative risk factors for survival.
Previous findings by a number of authors22,23 have shown that the tumor stage at initial presentation was the most important prognostic factor for survival in patients with NSCLC. Similarly, stage of disease was found to be an independent negative prognostic factor of survival in the present retrospective study.
The present study has got some limitations. First it is a retrospective study. Second, we did not evaluate the type of DM, duration of diabetes and the types of diabetic therapy used. Third, our findings may have a selection bias because of the possibilities of undiagnosed DM among patients classified as not having DM.
In conclusion, DM at the time of diagnosis was associated with the negative prognostic importance for PFS and OS in advanced stage patients receiving first-line platinum-based doublets chemotherapy. In addition, poor performance status and advanced stage were identified as negative prognostic factors. It may be concluded that these findings may also facilitate pretreatment prediction of survival and can be used for selecting patients for the correct choice of treatment. Therefore, a prospective trial and larger clinical trials are needed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.