The aim of this study was to evaluate the effectiveness of implementing a physical therapy guideline for patients undergoing upper abdominal surgery (UAS) in reducing the incidence of atelectasis and length of hospital stay in the postoperative period.
Materials and methodsA “before and after” study design with historical control was used. The “before” period included consecutive patients who underwent UAS before guideline implementation (intervention). The “after” period included consecutive patients after guideline implementation. Patients in the pre‐intervention period were submitted to a program of physical therapy in which the treatment planning was based on the individual experience of each professional. On the other hand, patients who were included in the post‐intervention period underwent a standardized program of physical therapy with a focus on the use of additional strategies (EPAP, incentive spirometry and early mobilization).
ResultsThere was a significant increase in the use of incentive spirometry and positive expiratory airway pressure after guideline implementation. Moreover, it was observed that early ambulation occurred in all patients in the post‐intervention period. No patient who adhered totally to the guideline in the post‐intervention period developed atelectasis. Individuals in the post‐intervention period presented a shorter length of hospital stay (9.2±4.1 days) compared to patients in the pre‐intervention period (12.1±8.3 days) (p<0.05).
ConclusionThe implementation of a physical therapy guideline for patients undergoing UAS resulted in reduced incidence of atelectasis and reduction in length of hospital stay in the postoperative period.
O objetivo deste estudo foi avaliar a eficácia da implementação de uma diretriz de fisioterapia para doentes submetidos a cirurgia abdominal superior (UAS) na redução da incidência de atelectasia e no tempo de internamento no pós‐operatório.
Materiais e MétodosFoi usado um desenho de estudo de “antes e depois com controlo histórico. O período “antes” incluiu doentes consecutivos que foram submetidos a UAS antes da implementação da diretriz (intervenção). O período “depois” incluiu doentes consecutivos após a implementação da diretriz. Os doentes no período pré‐intervenção foram submetidos a um programa de fisioterapia onde o planeamento do tratamento foi baseado na experiência individual de cada profissional. Por outro lado, os doentes que foram incluídos no período pós‐intervenção foram submetidos a um programa padronizado de fisioterapia com um foco no uso de estratégias adicionais (EPAP, espirometria de incentivo e mobilização precoce).
ResultadosOcorreu um aumento significativo do uso de espirometria de incentivo e pressão expiratória positiva nas vias aéreas após a implementação das diretrizes. Além disso, observou‐se que ocorreu o levantamento precoce em todos os doentes durante o período pós‐intervenção. Nenhum doente que aderiu totalmente à diretriz no período pós‐intervenção desenvolveu atelectasia. Os indivíduos no período pós‐intervenção apresentaram um menor tempo de internamento hospitalar (9.2±4.1 dias) em comparação com os doentes no período pré‐intervenção (12.1±8.3 dias) (p<0.05).
ConclusãoA implementação de uma diretriz de fisioterapia para doentes submetidos a UAS resultou na redução da incidência de atelectasia e na redução do tempo de internamento no pós‐operatório.
Postoperative pulmonary complications (PPCs) are common in patients undergoing abdominal surgery and are responsible for the increased morbidity and mortality as well as length of hospital stay and health related cost of care.1,2 The PPCs occur more frequently in surgeries where the incision is made above the umbilical scar, the so called upper abdominal surgeries (UAS).3 The incidence of PPCs in these subjects is related to the existence of preoperative risk factors such as advanced age, smoking, malnutrition, obesity, lung diseases, and clinical diseases. Surgical and anesthetic factors such as the time of surgery, type of surgery, and the effects of anesthetic drugs on the respiratory system also contribute to the development of PPCs.4
Atelectasis, pneumonia, acute respiratory failure, tracheobronchitis, wheezing, and prolonged mechanical ventilation are the most commonly observed PPCs.2 It is known that the decrease in lung volumes and capacities, abnormal respiratory pattern, abnormal gas exchange, and pulmonary defenses in patients undergoing open UAS start with anesthetic induction and perpetuate in the postoperative period, contributing to the occurrence of these PPCs.5,6 The respiratory muscle dysfunction has also been attributed to the development of PPCs.7,8 Multiple factors may be involved in diaphragmatic dysfunction, such as irritation and inflammation caused by trauma from manipulation close to the diaphragm, reflex inhibition of afferent abdominal receptors, and postoperative pain.7
In this context, physical therapy assistance to open UAS aims to preserve pulmonary function and reverse physiological and/or functional changes that may occur in the postoperative period due to these complications.9,10 Therefore, physical therapy provides a variety of interventions that must be individually selected according to the needs of the patient. Chest physical therapy acting with thoracic expansion exercises and diaphragmatic breathing exercises immediately after the UAS appears to improve oxygenation without triggering increase in pain or other complications.11 Furthermore, interventions that increase lung volume such as deep breathing exercises, incentive spirometry and continuous positive airway pressure (CPAP) are associated with lower frequency PPCs.12 However, the number of clinical studies that highlight the benefits of applying prophylactic therapy in patients undergoing open UAS is still quite limited.13,14
The main objective of the present study was to evaluate the effectiveness of the implementation of a guideline for physical therapy assistance for patients undergoing elective open UAS in reducing the incidence of atelectasis and length of hospital stay in the postoperative period.
Materials and methodsThe study was conducted in a private and tertiary care center of 497 beds in the state of São Paulo, Brazil. We analyzed data from patients hospitalized in intensive care units, semi‐intensive units, and wards. The study included adult patients (age≥18 years) undergoing elective open UAS and who received physical therapy in the postoperative period. It excluded patients undergoing lower abdominal surgeries, laparoscopic surgeries, emergency surgeries, surgeries with associated chest manipulation, those who underwent more than one surgical procedure during hospitalization, patients who did not adhere properly to the physical therapy treatment (performing physical therapy attendances <75% of scheduled therapy), patients who initiated inpatient physical therapy before the surgery (preoperative physical therapy), individuals who died during hospitalization, and patients requiring invasive mechanical ventilation over 24hours.
We used a “before and after” model of retrospective study with historical control.15,16 The “before” period (pre‐intervention) included all consecutive patients undergoing elective open UAS who met the criteria for inclusion in the study over six months (from July to December 2010) before guideline implementation (intervention). Teams of physical therapists were trained in the standardization of the new model of care during the month of January 2011. During this period, no data of patients undergoing UAS were collected. The “after” period (post‐intervention) included all consecutive patients who met the inclusion criteria of the study during the six months after guideline implementation (from February to July 2011).
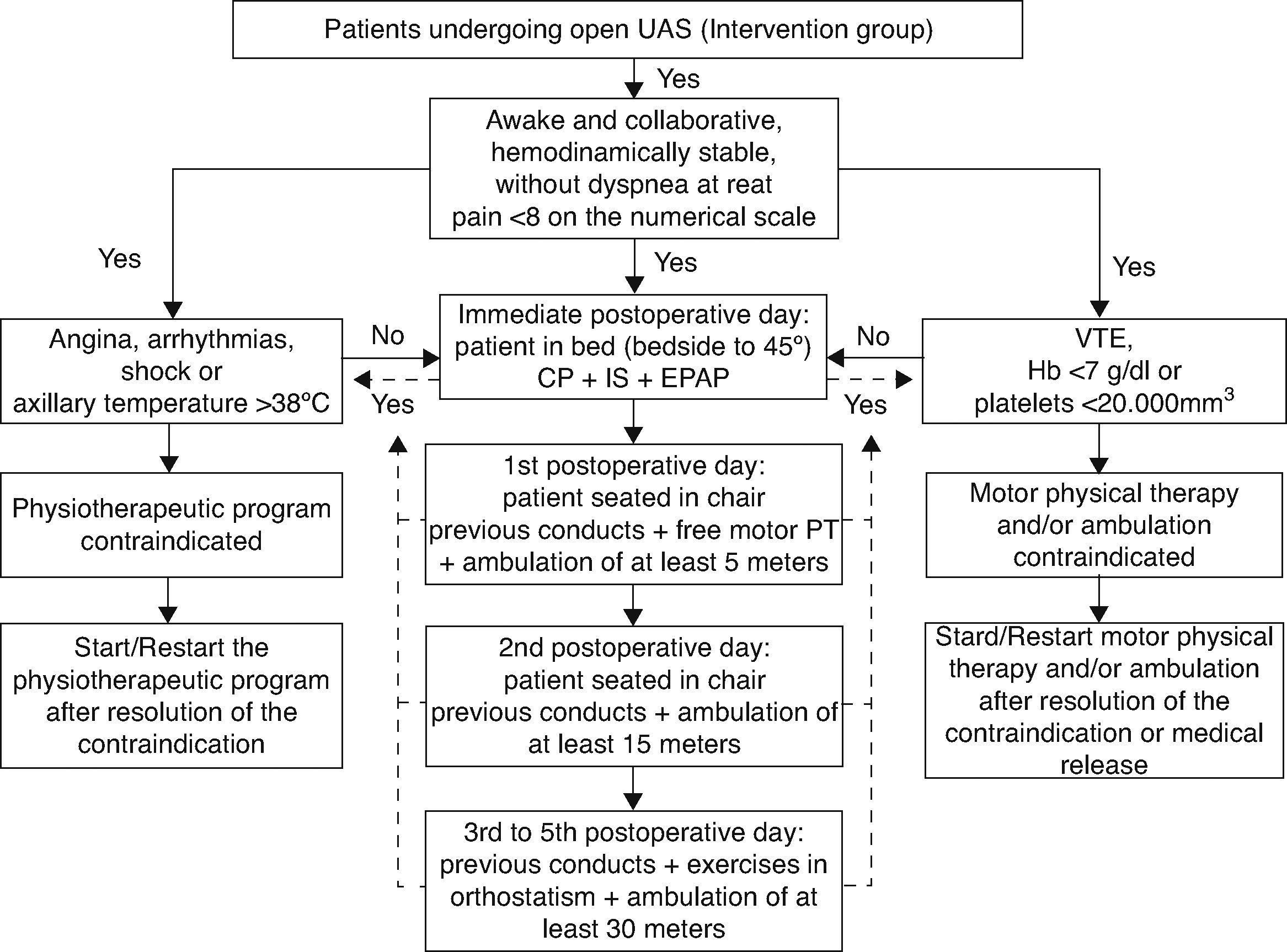
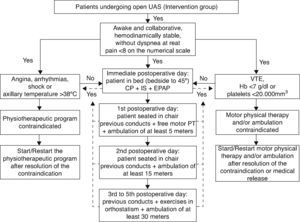
A training program for guideline implementation was carried out by the area of Continuing Education of the Rehabilitation Service of the institution for a period of 30 days. Fifteen training meetings were arranged in small groups for all the 126 physical therapists of the institution, acting in intensive care units, semi‐intensive units, and wards, in relation to the guideline. During the training sessions, we presented flow diagrams for treatment, the standardization of approaches of treatment, orientations for hospital discharge, and the scientific evidence that supported the elaboration of the guidelines. Furthermore, the training aimed to guide professionals in the use of physical therapy resources recommended in the care model (i.e. incentive spirometry and positive expiratory pressure in the airways). To disseminate the guideline, printed copies of the document in the operating units were distributed, in addition to providing the electronic file in the computerized system of the institution for consultations. This material contains information about care flowcharts, indications and contraindications, criteria for discontinuing the program, resources and frequency of physical therapy sessions (Fig. 1). The document presented a total of 11 pages including flowcharts.
Patients included in the study in the pre‐intervention period (control group) underwent a program of postoperative physical therapy treatment in which the therapeutic planning to be applied was determined by the professional providing patient care (non‐standard model). In contrast, patients who were included in the post‐intervention (intervention group) underwent a standardized program of physical therapy treatment which structured the model of patient care, focusing on the use of additional therapeutic resources (volumetric incentive spirometry and positive expiratory pressure in the airways),17,18 early sitting position and ambulation (onset <48h after surgery)19 (Fig. 1). Patients undergoing the program preconized by the guideline should undergo at least two sessions of physical therapy daily until the 5th postoperative day.13 Therapeutic treatment was discussed again and re‐planned by the team of physical therapists after the 5th postoperative day, to redefine the need for two sessions daily.
In the present study, total adhesion to the guideline was defined by the use of all the features recommended in the guideline. When one of the resources was not applied, it was considered partial compliance, and when two or more features were not used, it was considered as non‐adherence to the guideline.
Data were collected from the analysis of medical records and electronic database of the hospital. The information extracted from these sources was stored in electronic format previously designed for this study. We collected data concerning characterization of each patient (medical history, demographics, clinical and anthropometric data), the surgical procedure (type of surgery, surgical technique, surgical time, and surgical risk), and the physical therapy assistance provided to patients during hospitalization (features used and treatment adherence). Regarding the outcomes investigated, the incidence of atelectasis was considered as the primary variable and the length of hospital stay as the secondary variable.
The diagnosis of atelectasis was considered in the presence of imaging studies confirming this alteration. All patients included in the study, both in the control group (CG) and in the intervention group (IG), had radiographic evaluation from the first to fifth postoperative day. We considered only the presence of pure atelectasis (not associated with other complications such as pleural effusion or pneumothorax) since the aim of the study was to determine the incidence of atelectasis secondary only to the surgery, and not to other complications. Radiologists who had read all the examinations did not know the study objectives. We considered as possible risk factors for developing atelectasis: age, female gender, high body mass index (BMI), lung disease, history of smoking, hypertension, diabetes, dyslipidemia, heart disease, cancer, type of surgery, surgical technique, time of surgery, and surgical risk (American Society of Anesthesiologists scale).
The sample size calculation was performed based on a pilot study that showed a percentage of 25% for the incidence of atelectasis among patients undergoing UAS who were not submitted to the guideline. We also considered one previous study showing a reduction to a proportion of 6% of atelectasis among patients undergoing a protocol of physical therapy in the postoperative.20 With a 5% significance level and a power test of 90%, the sample was determined to be 66 patients for each group. To reach this number, we calculated that we needed 6 months for the post‐intervention period. For the final analysis, a sensitive analysis was performed including only the patients with full adherence to the guideline as well as an analysis of all patients included in the post‐intervention period. Categorical variables are presented as frequency, whereas continuous variables are expressed as mean±standard deviation. Comparisons between groups were made by Mann–Whitney test (nonparametric data), in the case of numerical variables, and by the chi‐square test in the case of categorical variables. The level of significance was 5%. Statistical analysis was performed using the statistical program SigmaPlot 11.0 (Systat Software Inc., CA, USA).
This study was approved by the Ethics Committee of the hospital (Registration number: HSL 2010‐58). There was waiver shall of the Consent Form, because it is a retrospective observational study analyzing standardization of institutional care process.
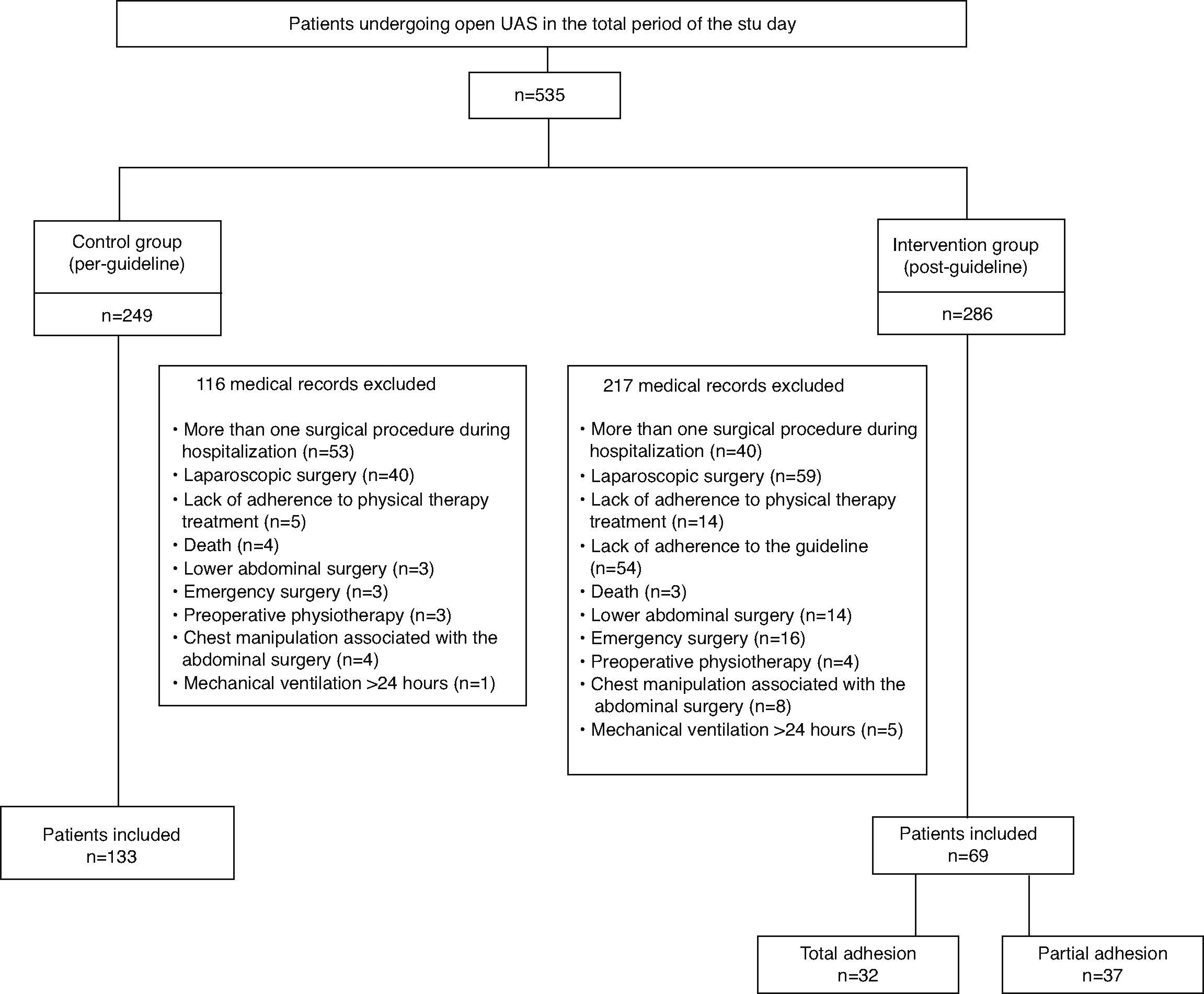
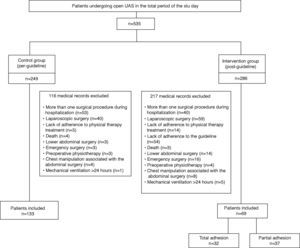
ResultsWe analyzed medical records of 535 patients undergoing UAS in the total period of the study, 249 belonging to the stage prior to guideline implementation and 286 belonging to the subsequent stage. After evaluation of inclusion and exclusion criteria, 202 were eligible for the study. The CG consisted of 133 patients and the IG of 69 patients (Fig. 2). Of the patients included in the IG, 32 (46.4%) had total adherence to the guideline, whereas 37 (53.6%) had partial adherence to the guideline (did not undergo one of the additional therapeutic resources in the proposed guideline).
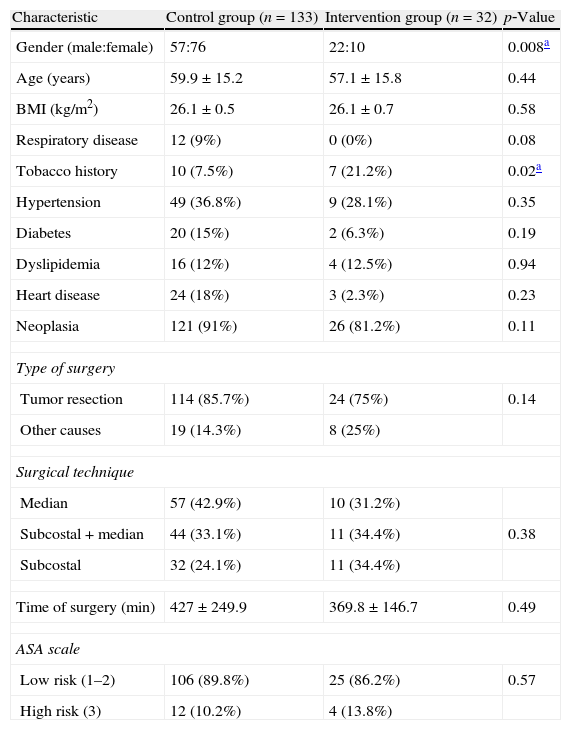
The clinical and demographic characteristics of the population studied in each group are presented in Table 1. There was significant difference between the groups only in relation to smoking history and gender. The IG patients presented a higher prevalence of tobacco use and a higher proportion of males. The main reason for the surgery was the presence of tumor with resection indication for both CG (91%) and IG (81.2%). There was no difference between groups in relation to surgical risk, technique or surgery time.
Demographic and clinical characteristics of individuals included in the study.
| Characteristic | Control group (n=133) | Intervention group (n=32) | p‐Value |
| Gender (male:female) | 57:76 | 22:10 | 0.008a |
| Age (years) | 59.9±15.2 | 57.1±15.8 | 0.44 |
| BMI (kg/m2) | 26.1±0.5 | 26.1±0.7 | 0.58 |
| Respiratory disease | 12 (9%) | 0 (0%) | 0.08 |
| Tobacco history | 10 (7.5%) | 7 (21.2%) | 0.02a |
| Hypertension | 49 (36.8%) | 9 (28.1%) | 0.35 |
| Diabetes | 20 (15%) | 2 (6.3%) | 0.19 |
| Dyslipidemia | 16 (12%) | 4 (12.5%) | 0.94 |
| Heart disease | 24 (18%) | 3 (2.3%) | 0.23 |
| Neoplasia | 121 (91%) | 26 (81.2%) | 0.11 |
| Type of surgery | |||
| Tumor resection | 114 (85.7%) | 24 (75%) | 0.14 |
| Other causes | 19 (14.3%) | 8 (25%) | |
| Surgical technique | |||
| Median | 57 (42.9%) | 10 (31.2%) | |
| Subcostal+median | 44 (33.1%) | 11 (34.4%) | 0.38 |
| Subcostal | 32 (24.1%) | 11 (34.4%) | |
| Time of surgery (min) | 427±249.9 | 369.8±146.7 | 0.49 |
| ASA scale | |||
| Low risk (1–2) | 106 (89.8%) | 25 (86.2%) | 0.57 |
| High risk (3) | 12 (10.2%) | 4 (13.8%) | |
BMI: body mass index; ASA: American Society of Anesthesiologists (surgery risk).
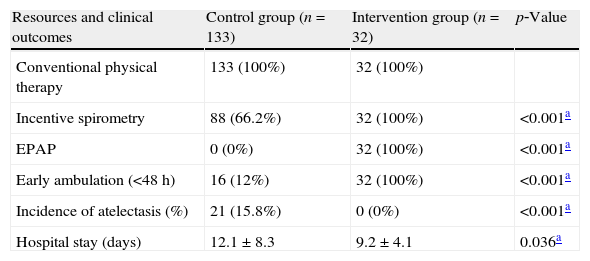
The guideline implementation optimized the use of additional therapeutic resources during physical therapy assistance, causing a significant increase in the use of incentive spirometry and expiratory positive airway pressure (EPAP) (p<0.001). Furthermore, it was observed that early ambulation occurred in all patients in the post‐intervention period. In contrast, only 12% of patients in the pre‐intervention period managed early ambulation (Table 2). Regarding the clinical outcomes, no patient in the IG showed pure atelectasis, whereas the frequency of atelectasis in the CG was 15.8% (n=21) with statistically significant difference between groups (p<0.05) (Table 2). When the intention‐to‐treat (ITT) analysis was performed (including patients with partial adherence to the guideline), the rate of atelectasis was 13% (n=9) without statistically significant difference compared to the CG (p=0.362). There was also difference between groups in the length of hospital stay. CG individuals remained hospitalized for a longer period of time (12.1±8.3 days) when compared to the IG (9.2±4.1 days) in the postoperative period (p<0.05) (Table 2). The ITT analysis also revealed that the length of hospital stay was still lower in the IG (10.8±6.4 days) when compared to the CG (12.1±8.3 days), but without statistically significant difference (p=0.24).
Frequency of use of different therapeutic resources and clinical outcomes (incidence of atelectasis and length of hospital stay).
| Resources and clinical outcomes | Control group (n=133) | Intervention group (n=32) | p‐Value |
| Conventional physical therapy | 133 (100%) | 32 (100%) | |
| Incentive spirometry | 88 (66.2%) | 32 (100%) | <0.001a |
| EPAP | 0 (0%) | 32 (100%) | <0.001a |
| Early ambulation (<48h) | 16 (12%) | 32 (100%) | <0.001a |
| Incidence of atelectasis (%) | 21 (15.8%) | 0 (0%) | <0.001a |
| Hospital stay (days) | 12.1±8.3 | 9.2±4.1 | 0.036a |
EPAP: expiratory positive airway pressure.
Table 3 describes possible risk factors for the development of atelectasis. These data are related only to the CG, since there was no development of atelectasis in the IG. In the present study, the only risk factor associated with the development of atelectasis was the surgical technique. The individuals undergoing subcostal incisions were more likely to develop this complication (p<0.05).
Possible risk factors associated with the development of atelectasis.
| Characteristic | With atelectasis (n=21) | Without atelectasis (n=112) | p‐Value |
| Gender (male:female) | 11:10 | 65:47 | 0.63 |
| Age (years) | 63.1±13.5 | 59.2±15.5 | 0.27 |
| BMI (kg/m2) | 26.9±4.4 | 26.5±5.5 | 0.14 |
| Respiratory disease | 3 (14.3%) | 9 (8%) | 0.36 |
| Tobacco history | 3 (14.3%) | 7 (6.2%) | 0.2 |
| Hypertension | 8 (38%) | 41(36.6%) | 0.9 |
| Diabetes | 3 (14.3%) | 17 (15.2%) | 0.92 |
| Dyslipidemia | 2 (9.5%) | 14 (12.5%) | 0.7 |
| Heart disease | 5 (23.8%) | 19 (17%) | 0.45 |
| Neoplasia | 18 (85.7%) | 95 (84.8%) | 0.92 |
| Type of surgery | |||
| Tumor resection | 18 (85.7%) | 96 (85.7%) | 1 |
| Other causes | 3 (14.3%) | 16 (14.3%) | |
| Surgical technique | |||
| Median | 5 (23.8%) | 52 (46.4%) | |
| Subcostal+median | 5 (23.8%) | 39 (34.8%) | 0.004a |
| Subcostal | 11 (52.4%) | 21 (18.8%) | |
| Time of surgery (min) | 474±306.3 | 419.7±239 | 0.68 |
| ASA scale | |||
| Low risk (1–2) | 16 (76.2%) | 90 (90.9%) | 0.38 |
| High risk (3) | 3 (15.8%) | 9 (9.1%) | |
| Late ambulation | 10 (47.6%) | 33 (29.5%) | 0.12 |
BMI: body mass index; ASA: American Society of Anesthesiologists (surgery risk).
The present study showed that the optimization and standardization of the use of additional therapeutic resources through the implementation of a guideline for physical therapy assistance, guiding the care of patients undergoing UAS, is effective in reducing the incidence of atelectasis and length of hospital stay in the postoperative period.
Previous studies have reported that the incidence of atelectasis observed in the postoperative period can vary from 6% to 42%.21,22 In the present study, the incidence of atelectasis among the CG patients (pre‐intervention period) was 15.8%, which is consistent with these previous reports. The optimization of physical therapy treatment in the IG (post‐intervention) reduced the incidence of atelectasis to 0% in those patients who adhered totally to the guideline, highlighting the importance of the adequacy of physical therapy in the postoperative care. The intention‐to‐treat analysis (including 37 patients who adhered partially to the guideline) did not show a statistically significant difference in the rate of atelectasis or length of hospital stay when compared to the CG. This finding reinforces the need for total adherence of the intervention packages for the clinical outcomes to be achieved. Although the ITT analysis did not demonstrate a statistically significant difference for the length of hospital stay, a reduction of up to 48h in hospitalization can be considered clinically relevant and can be associated with a reduction in healthcare costs. In association with these findings, it was also observed that the length of hospitalization was higher among patients who developed atelectasis. The longer length of stay among patients who develop pulmonary complications is a common finding in the literature.2
Although physical therapy assistance is routinely used in the processes of functional rehabilitation of patients undergoing UAS,11 the results demonstrating its effectiveness in preventing atelectasis are still inconsistent.13 The absence of consolidated scientific evidence can lead the therapist to carry their professional practice using clinical decisions based on their own experience, which results in a wide range of care practices in a service.23 Standardizing practical approaches becomes necessary to help teams to make the most appropriate decision, favoring the clinical outcomes of patients. In this context, the development of care guidelines has been widely used in the routine in different fields of medical activity,15,16 providing practical recommendations when scientific evidence is still limited or questionable.23
Therapeutic resources such as incentive spirometry, CPAP, EPAP, early mobilization, and conventional physical therapy, based on deep breathing exercises, are often used to prevent atelectasis in patients undergoing UAS.24 Among these commonly used features, incentive spirometry appears to be involved in more controversy. Recent systematic reviews have found no evidence regarding the effectiveness of the use of incentive spirometry for preventing pulmonary complications in the postoperative period of UAS.25,26 However, many of the studies investigating the effectiveness of this device still present methodological flaws, making the elaboration of more rigorous studies necessary to define the real benefits of the use of incentive spirometry. Despite these inconsistent results, the latest recommendations on the use of incentive spirometry in preventing postoperative pulmonary complications indicate that this feature should be applied in combination with deep breathing techniques, assisted cough, early mobilization, and optimized analgesia to obtain better preventive results.27 In the present study, incentive spirometry was used in combination with other techniques recommended for postoperative, which probably contributed to the reduction of the incidence of atelectasis.
Another feature preconized for prophylaxis of atelectasis in patients undergoing UAS is the use of breathing exercises associated with positive pressure through EPAP or CPAP.9 Although the use of CPAP is a strategy recommended for prophylaxis of atelectasis for UAS,9 its application in clinical practice is quite limited by the risk of abdominal distension related to aerophagia, which can be particularly harmful in the occurrence of fistulas or anastomosis leakage. Ricksten et al.18 have demonstrated that both the use of CPAP and EPAP were effective in preserving lung volumes and preventing the development of atelectasis in the postoperative period of abdominal surgeries, and that the use of these resources were superior to deep breathing exercises. Other authors have also demonstrated that EPAP is as effective as CPAP for the prevention of PPCs after thoracic surgery and should be used concomitantly with conventional respiratory physical therapy.28,29 In the present study, it was found that in the period before guideline implementation EPAP was not a strategy used in routine. In the post‐intervention period, EPAP was used in all patients who adhered to the guideline. The reduction in the rate of atelectasis may have been largely explained as a result of the inclusion of this feature in clinical practice.
Finally, early mobilization was another important feature recommended in the approach of patients undergoing open UAS after guideline implementation. It is believed that early mobilization results in increased lung volume, with consequent prevention of atelectasis.30 Brasher et al.31 have even suggested that early mobilization seems to be more effective than deep breathing exercises for the prevention of PPCs. These findings further emphasize the importance of early mobilization in the postoperative period for UAS.
Risk factors such as age over 60, smoking history, presence of chronic lung disease and surgical time over 210min are often related to the occurrence of pulmonary complications in the postoperative period of open UAS.4 Interestingly, the only risk factor associated with the development of atelectasis, in the present study, was the surgical technique, with patients undergoing subcostal incisions presenting higher incidence of complications. The relationship between subcostal incisions and the development of pulmonary complications after abdominal surgery has been previously demonstrated.3
The main limitation of the present study relates to the methodological design. Although the use of a historical control hinders the establishment of a causal association, its application in an institutional context becomes a more viable alternative. It should also be noted that the implementation of the guideline was the only change incorporated into the care of these patients during the study period. Another factor worth mentioning is the fact that radiological assessors were blinded to the study objectives ensuring the reliability of the diagnosis of atelectasis (primary variable). Moreover, the short time between the historical control and the intervention period strengthens the assumption of a true association between the interventions and the observed outcomes. Another limitation of the present study was the difference in the sample size of patients undergoing the guideline in the post‐intervention period compared to the pre‐intervention period. It was observed that the reduction in the number of patients included in the post‐intervention period was mainly for the accuracy of the selection criteria and a poor adherence to the guideline by the physical therapists. However, poor adherence in periods immediately after the implementation of healthcare guidelines was also observed in previous studies.15,32 This fact emphasizes the need for continuing education work for the consolidation of long‐term processes.
ConclusionsIn the present study, the efficacy of isolated conventional physical therapy, incentive spirometry, EPAP or early mobilization was not evaluated. However, it was possible to demonstrate that the physiotherapeutic approach based on packages of interventions resulted in reduced incidence of atelectasis and reduced length of hospital stay among patients undergoing elective open UAS. The verification of these favorable outcomes strengthens initiatives for the development of other physical therapy practices based on managed guidelines, providing foundation for the awareness of the teams on the importance of following these care models.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.