It is widely recognized that asthma control is not always possible in patients with very severe asthma despite available treatment. The aim of this study was to evaluate the efficacy of Omalizumab on asthma control as an add‐on therapy in patients from the “Severe Asthma Outpatient Service” of Pulido Valente Hospital in Lisbon, Portugal.
MethodsA retrospective study was conducted to assess asthma control by the ACT score and by GINA classification, frequency and severity of exacerbations, medication use and pulmonary function in patients treated with Omalizumab. Clinical information was collected from medical records from the start of treatment and at 6‐, 12‐ and 24‐month follow‐ups.
Results26 patients started the treatment with Omalizumab, and all (100%) were classified by GINA with uncontrolled asthma prior to treatment. Mean ACT score was 11.5. All the patients had treatment with fixed‐dose ICS and LABA and 34.6% also had an anti‐cholinergic inhaler. 42.3% of patients were also treated with oral glucocorticosteroids for control. Patients reported an average of 1.8 moderate and 3.1 severe exacerbations/year. Statistical differences were found at 6‐month follow‐up in most end‐points: GINA score improved: 60.9% of patients with partially controlled asthma and only 39.1% with uncontrolled asthma (Wilcoxon 0.00); ACT score improved to 19.52 (Wilcoxon 0.00); mean FEV1 improved to 76.7% (Wilcoxon 0.025); the proportion of patients requiring oral glucocorticosteroid therapy reduced to 17.4% (Wilcoxon 0.014); and the number of moderate and severe exacerbations also decreased to 1.04 and 1.83 respectively (Wilcoxon 0.007; Wilcoxon 0.002 respectively).
ConclusionsThe current analysis shows evidence that omalizumab is successful in improving asthma control as an add‐on therapy GINA step 5 treatment.
Está bem documentado que o controlo de asma nem sempre é possível em doentes com asma grave apesar da terapêutica otimizada. O objetivo deste estudo foi avaliar a eficácia de omalizumab no controlo de asma como terapêutica adjuvante em doentes seguidos na consulta de asma grave do Hospital Pulido Valente, em Lisboa.
MétodosRealizámos um estudo retrospetivo que avaliou o controlo de asma quantificado pelo score ACT e pela classificação GINA, a frequência e gravidade das exacerbações, a medicação em curso e a função pulmonar nos doentes tratados com omalizumab. A informação clínica foi obtida através dos registos clínicos da consulta nos doentes submetidos a esta terapêutica, na fase inicial do tratamento e aos 6, 12 e 24 meses de seguimento.
ResultadosVinte e seis doentes iniciaram terapêutica com omalizumab, todos com asma não controlada pela classificação GINA antes do tratamento com uma média de ACT 11.5. Todos os doentes estavam medicados com doses fixas de ICS e LABA e 34,6% estavam igualmente medicados com inalador anticolinérgico. 42,3% dos doentes também estavam medicados com corticoides orais de forma mantida. Os doentes reportavam uma média de 1,8 e 3,1 exacerbações moderadas e graves por ano, respetivamente. Diferenças significativas foram demonstradas no seguimento aos 6 meses na maioria dos parâmetros em estudo com melhoria do score GINA: 60,9% dos doentes passaram a ter asma parcialmente controlada e apenas 39,1% mantiveram asma não controlada (Wilcoxon 0,00); subida do score ACT para 19,52 (Wilcoxon 0,00); melhoria da média de FEV1 para 76,7% (Wilcoxon 0,025); descida na proporção de doentes a necessitar corticoterapia sistémica para 17,4% (Wilcoxon 0,014); e redução do número de exacerbações moderadas e graves para 1,04 e 1,83 por ano, respetivamente.
ConclusõesEste estudo vem demonstrar que o omalizumab é eficaz no controlo de asma como terapêutica adjuvante da asma (GINA degrau 5).
It is well known that asthma control is not always achieved in patients with more severe asthma despite available treatment. Global Initiative for Asthma (GINA) 2011 guidelines recommend an approach for achieving the best possible results in terms of symptoms, rescue medication use and lung function in order to reduce the risk of exacerbations and death related to asthma. Severe asthma is defined as asthma that requires high intensity treatment to maintain good control or where good control is not achieved despite high intensity treatment.1 It is estimated that approximately 5% of asthma patients have severe asthma.2 However, a significant number of patients are poorly controlled despite combination therapy with high doses of inhaled corticosteroid (ICS) and long‐acting B‐agonist (LABA) and leukotriene antagonists. The GOAL study demonstrated that 38–53% of patients using “optimal therapy” remain poorly controlled.3 These patients suffer frequently from asthma symptoms with a major impact on their daily activities and often experience exacerbations with multiple hospital visits.1 Omalizumab is the first licensed anti‐immunoglobulin (Ig) E antibody shown to be effective for the treatment of allergic (IgE‐mediated) asthma. The Global Initiative for Asthma (GINA) 2011 guidelines recommend omalizumab as an add‐on step 5 treatment to fixed dose ICS and LABA combination therapy. Many recent studies demonstrate the efficacy of Omalizumab in achieving control in patients with severe allergic asthma. In the INNOVATE study Omalizumab reduced asthma exacerbation rate by 26% and halved the severe exacerbation rate in patients treated with high dose ICS and LABA, with reduced lung function and a history of clinically significant exacerbation in the past year. Emergency visits were reduced by 44% compared to placebo. Omalizumab has also been found to significantly improve asthma‐related QoL, asthma symptom scores, and lung function.2,4 The ongoing prospective study EXCELS with 5000 patients treated with Omalizumab has already shown that initiation of omalizumab treatment was associated with decreased doses of ICS, short acting B‐agonists (SABA) Leukotriene receptor antagonists (LTRA) at 2 years follow‐up.5 Studies have also shown a steroid sparing effect with Omalizumab, which reduces adverse side‐effects caused by oral corticosteroids.3,6 Safety and tolerability have been consistent in published data for Omalizumab.2,3,7
The Pulido Valente Hospital in Lisbon is one of the largest Pulmonary Medicine centers in Portugal. Difficult to treat Asthma patients are referred to the “Severe Asthma Outpatient Unit” at the hospital for follow‐up with pulmonary medicine and allergy‐immunology experts. Omalizumab was first used in this unit in 2006 in selected patients with severe, non‐controlled asthma despite optimal treatment.
The aim of this study was to evaluate the efficacy of Omalizumab on asthma control since its introduction as an add‐on therapy on patients from the “Severe Asthma Outpatient Unit” of Hospital Pulido Valente, Lisbon.
MethodsThis was an observational study to determine the clinical control of asthma, frequency and severity of exacerbations, control medication required and pulmonary function in patients being treated with Omalizumab from the “Severe Asthma Outpatient Unit”. Information was collected from patient clinical records dating from the year prior to initiation of Omalizumab and at 6‐, 12‐ and 24 months of treatment. Asthma control was determined by applying the ACT score to information collected from medical records as well as classification of asthma control according to GINA guidelines. Exacerbations were classified as moderate or severe according to ATS recommendations for asthma clinical trials.8 The rate (number per year) and severity of exacerbations were recorded at each time interval. Ongoing asthma medication was also recorded at each time interval. As this was a retrospective study it was not possible to consistently have lung function tests at all time intervals of this study. Lung function tests were ordered according to the physician's discretion. FEV1 results were collected when available. Data were analyzed using the SPSS 15 version. Data at 6‐, 12‐, and 24 months were individually statistically compared with the patients’ baseline characteristics. Data were also checked for statistical differences between each time interval. Mean ACT scores, classification of asthma control according to GINA, mean number of moderate and severe exacerbations per year, and ongoing maintenance therapy were compared and tested for statistical differences using Wilcoxon test for paired variables. Mean FEV1 was tested for normality distribution which allowed FEV1 to be tested for statistical differences using Student's t‐test for the 12‐ and 24‐month intervals compared to baseline results. Due to small sample size mean FEV1 at the 6‐month interval was tested for statistical differences to baseline results with Wilcoxon test.
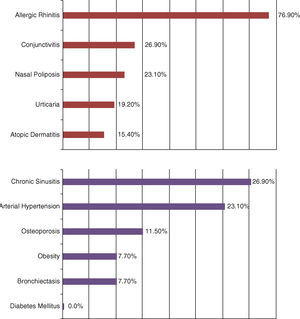
ResultsA total of 26 patients started the treatment with Omalizumab (Table 1). All patients had been optimally treated with standard therapy by physicians of this unit for at least 1 year before being considered for the treatment with Omalizumab. Dosage of Omalizumab was given according to weight and serum IgE. 23 patients have reached 6‐ and 12‐month follow‐ups after initiating treatment and 13 patients have had follow‐up for 24 months. 1 patient abandoned treatment with Omalizumab at 12‐month follow‐up due to change of residence. None abandoned therapy due to adverse events. The demographic and background characteristics are as shown in Table 1. Most were female Caucasians, non‐smokers…3 patients had stopped smoking >10 years before (15, 15 and 10 pack‐years, respectively). Other relevant data from personal history are shown in Fig. 1. It is important to note the high incidence of allergic rhinitis in this population (76.9%) and other allergy related diseases. None of the patients with osteoporosis could be weaned off maintenance from oral corticosteroids prior to Omalizumab which clearly shows the deleterious effect of this treatment.
Demographic and background characteristics of patients.
| N | 26 |
| Sex | |
| Female, % | 73.1% |
| Age | |
| mean (SD) | 53 (13.6) |
| Race | |
| Caucasian, % | 100% |
| Weight kg | |
| mean (SD) | 81.8kg (15.1) |
| Smoking history, % | |
| Never smoked | 88.5% |
| Ex‐smoker | 11.5% |
| FEV1 (% of predicted) | |
| Mean (SD) | 66.7% (19.1) |
| Serum total IgE (IU/mL) | |
| Mean (SD) | 1607 (1211) |
| Duration of Asthma, years | |
| Mean (SD) | 28 (13.1) |
| Allergies | |
| House mites | 76.9% |
| Molds | 23.1% |
| Pollens | 73.1% |
| Grasses | 46.2% |
| Cockroach | 11.5% |
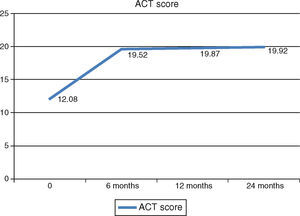
All patients had positive skin prick test to at least one perennial aeroallergen. Serum IgE level varied from 28.5 to 534IU/mL (mean 160.7IU/mL). Mean FEV1 was 66.7% before treatment. All patients were being treated with moderate to high dose ICS and LABA, and 42.3% had maintenance oral corticosteroids at baseline. Despite medication, all patients had had at least 1 moderate or severe exacerbation within 12 months prior treatment. Asthma control at baseline was classified as non‐controlled in all patients at baseline and mean ACT score was 12.08.
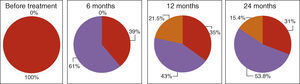
Asthma controlAsthma control as measured with the ACT score and GINA classification improved after initiation of Omalizumab. GINA asthma control improved significantly at 6‐month follow‐up with an increase in the number of patients with partially controlled asthma to 60.9%. After this initial improvement, there were no further significant improvements in asthma control at 12‐ and 24‐month follow‐ups. 15% of patients had their asthma completely controlled at 24‐month follow‐up (Fig. 2). ACT score had a similar progression, with an initial significant increase to a mean score of 19.52 at 6‐month follow‐up. Although there were no further significant improvements in ACT score at 12‐ and 24‐month follow‐ups, patients maintained asthma control at a consistent level which is portrayed as a plateau phase in asthma control with Omalizumab in Fig. 3.
Asthma control with Omalizumab – GINA classification. Red: non‐controlled, purple: partially controlled; orange: controlled asthma. Statistical differences were found at 6 months follow‐up from baseline (Wilcoxon 0.00).(For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
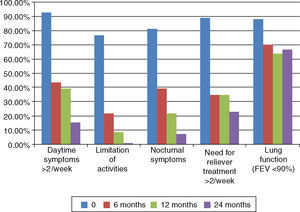
Analyzing the individual features of asthma control showed that patients with daytime symptoms>2× a week, limitation of activities, nocturnal symptoms and need for relief medication>2× a week, all reduced progressively after initiation of Omalizumab (Fig. 4). Significant changes were found at 6‐month follow‐up (Wilcoxon<0.01). Further significant differences were found between 12‐ and 24‐month follow‐ups. A significant decrease in the percentage of patients with daytime symptoms>2× a week and need for relief medication (Wilcoxon<0.05) and between 6‐ and 24‐month follow‐ups in limitation of activities and nocturnal symptoms (Wilcoxon<0.05).
Individual features of asthma control (GINA) at baseline and 6‐, 12‐ and 24‐month follow‐ups after initiation of Omalizumab. Daytime symptoms>2× a week: 92.3%, 43.5%, 39.1%, 15.4%; limitation of activities: 76.9%, 21.7%, 8.7%, 0%; nocturnal symptoms: 80.1% 39.1%, 21.7%, 7.7%; need for relief medication>2× a week: 88.5%, 34.8%, 34.8%, 23.1%; lung function FEV1<80%: 88%, 70%, 63.6%, 66.7%. Statistical differences are shown with *.
Lung function tests were available for 25 of the patients at baseline. At 6‐, 12‐ and 24‐month follow‐ups 9, 11 and 8 patients had these repeated lung‐function tests, respectively. The small number of lung‐function tests available makes it difficult to compare results in this assay and results must be interpreted accordingly. The percentage of patients with FEV1<90% decreased after initiation of Omalizumab. Statistical differences were only found at 12‐month follow‐up compared to baseline (Fig. 4). Mean value of FEV1 also improved and statistical differences were found at 6‐ and 12‐month follow‐ups when compared to baseline. Patients who repeated lung function tests at 6‐month follow‐up had a baseline FEV1 of 57.07% and had a significant improvement to 76.77% (Wilcoxon 0.025). Patients with reevaluation of FEV1 at 12‐month follow‐up had a baseline FEV1 of 58.61% and showed a significant improvement to 73.9% (p<0.05). No significant changes were found at 24‐month follow‐up.
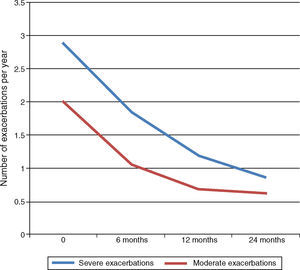
ExacerbationsMean number of exacerbations per year decreased after initiation of Omalizumab. Severe exacerbations, initially 3.13/year, fell significantly to 1.83 at 6‐month follow‐up and 1.7/year at 12‐month follow‐up (Wilcoxon<0.05). Further decrease at 24‐month follow‐up to 0.84/year was not significant. Moderate exacerbations also decreased significantly at 6‐ and 12‐month follow‐ups from 1.83/year at baseline to 1.04/year and 0.7/year respectively (Wilcoxon<0.05). Further decrease to 0.62/year at 24‐month follow‐up was not significant (Fig. 5).
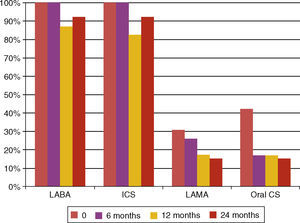
Maintenance therapyAt baseline 42.3% patients had maintenance therapy with oral corticosteroids. A significant decrease to 17.4% in the maintenance therapy with oral corticosteroids was found at 6‐month follow‐up (Wilcoxon 0.014) and this change continued at 12‐ and 24‐month follow‐ups. There was also a significant decrease in maintenance therapy with theophylline from 69.2% to 34.8% at 12‐month follow‐up (Wilcoxon 0.011). Regarding inhaled corticosteroid therapy we have observed: (a) an initial decrease in this treatment as 17.4% had no need of this therapy at 12‐month follow‐up (Wilcoxon 0.046), however this trend did not persist at 24‐month follow‐up (Fig. 6); (b) the initial mean dose at baseline was 1941.35μg/day of beclometasone dipropionate or equivalent and at 6‐, 12‐ and 24‐month follow‐ups the mean doses changed to 1576.92μg/day, 1600μg/day and 1850μg/day, respectively. Only at 6‐month follow‐up were these changes significant (Wilcoxon 0.02) and this is also reflected in the step‐down approach of other control medications, namely oral corticosteroids and theophyline.
Adverse eventsPain at the injection site was recorded in two patients. In one case it was only in the first administration and the other in the first two administrations. Both events subsided spontaneously with no need for therapeutic intervention. No other adverse events were reported.
ConclusionsThis study reflects published evidence that omalizumab is effective in improving asthma control as an add‐on therapy GINA step 5 treatment. Asthma control and pulmonary function as measured with FEV1 improved, frequency of moderate and severe exacerbations decreased and oral glucocorticosteroid therapy all reduced significantly with therapy with Omalizumab. Most significant changes were found at 6‐month follow‐up with some further improvement at 12 and 24 months follow‐up.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Alfarroba S, Videira W, Galvão‐Lucas C, Carvalho F, Bárbara C. Experiência clínica com Omalizumab na Consulta de Asma Grave. Rev Port Pneumol 2014;20:78–83.