The main feature of pulmonary emphysema is airflow obstruction resulting from the destruction of the alveolar walls distal to the terminal bronchioles. Existing clinical approaches have improved and extended the quality of life of emphysema patients. However, no treatment currently exists that can change the disease course and cure the patient. The different therapeutic approaches that are available aim to increase survival and/or enhance the quality of life of emphysema patients. In this context, cell therapy is a promising therapeutic approach with great potential for degenerative pulmonary diseases. In this protocol proposition, all patients will be submitted to laboratory tests, such as evaluation of heart and lung function and routine examinations. Stem cells will be harvested by means of 10 punctures on each anterior iliac crest, collecting a total volume of 200mL bone marrow. After preparation, separation, counting and labeling (optional) of the mononuclear cells, the patients will receive an intravenous infusion from the pool of Bone Marrow Mononuclear Cells (BMMC). This article proposes a rational and safe clinical cellular therapy protocol which has the potential for developing new projects and can serve as a methodological reference for formulating clinical application protocols related to the use of cellular therapy in COPD. This study protocol was submitted and approved by the Brazilian National Committee of Ethics in Research (CONEP – Brazil) registration number 14764. It is also registered in ClinicalTrials.gov (NCT01110252).
O enfisema pulmonar apresenta como principal característica a obstrução do fluxo aéreo resultante da destruição das paredes alveolares distais ao bronquíolo terminal. As abordagens terapêuticas clínicas existentes têm contribuído para o prolongamento e melhora na qualidade de vida dos portadores de enfisema. Porém, até o momento nenhum tratamento clínico existente tem sido capaz de alterar o curso da doença e resultar em cura do doente. As diferentes estratégias terapêuticas têm como objetivo o aumento da sobrevida e/ou a melhora na qualidade de vida dos doentes com enfisema pulmonar. Neste contexto, a terapia celular apresenta‐se como uma alternativa terapêutica promissora, com grande potencial de aplicabilidade em doenças degenerativas do pulmão. Nesta proposta de protocolo, todos os pacientes serão submetidos a testes laboratoriais, como a avaliação das funções cardíaca e pulmonar e exames de rotina. A colheita das células‐tronco será realizada por meio de 10 punções em cada crista ilíaca, totalizando um volume final de 200mL de medula óssea coletada. Após a preparação, separação, contagem e marcação (opcional) das células mononucleares, os pacientes receberão uma infusão intravenosa de uma suspensão de Células Mononucleares da Medula Óssea (CMMO). Pretende‐se, neste artigo, a proposta de um protocolo de terapia celular racional e seguro com potencial para o desenvolvimento de novos projetos, que sirva como referencial metodológico para a formulação de protocolos de aplicação clínica relacionados ao uso de terapia celular para a DPOC. O protocolo proposto neste artigo foi submetido e aprovado pelo Comitê Nacional de Ética em Pesquisa (Conep – Brasil) registado como o número 14.764. Também está registado no Clinical Trials.gov (NCT01110252).
Pathological states characterized by gradual, nonreversible airflow impairment come under the general heading of chronic obstructive pulmonary disease (COPD).1–3 Within the COPD spectrum, there are two nosological entities: chronic bronchitis and emphysema. Chronic bronchitis is characterized by fibrosis, luminal plugs, increased airways resistance and airways inflammation. The main feature of pulmonary emphysema is the destruction of the alveolar walls distal to the terminal bronchiole, without significant pulmonary fibrosis.1–6
The treatment of COPD and emphysema includes bronchodilators, short‐acting and long‐acting, β2‐agonists, anticholinergics, xanthines, corticosteroids, mucolytics and antibiotics. Other clinical support measures include rehabilitation therapies, oxygen therapy and ventilation strategies. New clinical and pharmacological approaches have significantly prolonged and improved the quality of life of patients with emphysema; however, there are no effective or curative treatments. Surgical treatment, such as lung transplantation, is an option which is theoretically effective; however, it is a high risk procedure and involves complex surgery; in addition it is severely affected by the scarcity of donors.1–8
In this context, cell therapy with adult stem cells (ASC) has not been thoroughly explored or studied, despite its great potential for the treatment of pulmonary degenerative diseases. A large number of previous studies in animal models have suggested that adult stem cells (hematopoietic and mesenchymal) would be able to migrate to injured areas and promote morphological and/or functional regeneration of the pulmonary parenchyma.9–12
The main objective of this article is to propose a rational and safe clinical cell therapy protocol employing Bone Marrow Mononuclear Cells (BMMC). This new methodological approach is expected to represent a potentially interesting and consistent reference for the development of new protocols for clinical application in COPD.
The protocol proposed in this article refers to the project registered in ClinicalTrials.gov (NCT01110252) and the Brazilian National Committee of Ethics in Research (CONEP – Brazil) – registration number 14764.
Methods/designStudy designThis study corresponds to a phase I clinical trial (safety evaluation of the procedure) with methodological and operational support from the University of the State of São Paulo – UNESP (Assis, SP, Brazil). The participants received written and verbal information explaining the study and written consent was obtained from all participants before beginning the procedure.
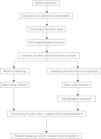
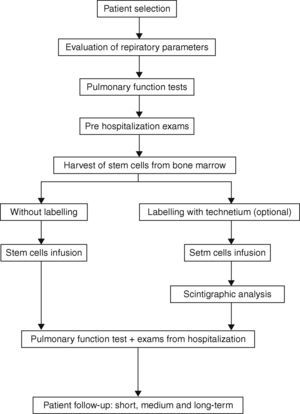
The protocol design was based on previous studies that have indicated that the procedures for collection, separation and infusion of BMMC are virtually free of adverse effects.11–17 The selection criteria were based on the fact that the patients, despite being in an advanced stage of COPD, presented good clinical, laboratory and psychological conditions (cardiac function, nutritional condition, psychosocial and emotional profile and family support) (Fig. 1).
Patient selectionInclusion criteriaTo be eligible to enroll, participants should (1) be aged between 40 and 76 years; (2) have severe obstructive pulmonary disease; (3) be limited in daily physical activities, Dyspnea Scale Score greater than 3, and other symptoms despite maximal clinical treatment; (4) have limited life expectancy; (5) have undergone pulmonary rehabilitation physiotherapy; (6) have an acceptable nutritional status; (7) have acceptable cardiac function; (8) not have used any tobacco products for at least six months; (9) have a satisfactory psychosocial and emotional profile and family support and (10) be capable of giving informed written consent.
Dyspnea Scale ScoreThe Dyspnea Scale Score, modified according to the British Council of Medical Research (Modified Medical Research Council Dyspnea Scale – MRC),18,19 is derived as follows:
- 1
Absence of dyspnea, except during extreme exertion.
- 2
Dyspnea when walking quickly on flat surfaces and/or when ascending a slight slope.
- 3
Walking more slowly on a flat surface due to dyspnea than a person of the same age, or having to stop when walking at a normal pace on a flat surface.
- 4
Stopping to breathe after walking one block (90–120m) or after walking a few minutes on a flat surface.
- 5
Dyspnea keeps the patient from leaving the house, or occurs during dressing or undressing.
Participants must be excluded if they have (1) active pulmonary or extra‐pulmonary infection; (2) serious cardiac pathology and/or ventricular dysfunction; (3) significant renal dysfunction and/or hepatic pathology, including hepatitis B and C; (4) detected immunosuppressive illnesses, including HIV; (5) smoking habits; (6) known neoplasias; (7) pregnancy or potential to become pregnant; (8) noncompliance with established medical protocol; (9) psychosocial problems, including drug or alcohol abuse; and (10) lack of family support.
Clinical evaluation and complementary laboratory tests in the pre‐processing phaseParticipants will initially undergo a complete evaluation of their pulmonary function. This evaluation will include clinical examination, spirometry before and after bronchodilator inhalation of salbutamol+ipratropium for maximum reversibility and measurement of lung volumes and airflow, especially the forced expiratory volume in one second (FEV1), the vital capacity (VC), forced vital capacity (FVC) and its ratio (FEV1/VC and FEV1/FVC). Total lung capacity, residual volume and pulmonary diffusion capacity, and arterial blood gases will be determined and CXR performed. Cardiac evaluation including ECG, echocardiography and cardiopulmonary exercise testing, will be performed. Further imaging will include CT scan – chest (helical/high resolution), scintigraphic ventilation/perfusion. Routine tests (CBC, coagulation, BUN, liver and bone profile, fasting glucose, creatinine, CRP (C‐reactive protein), and serum tests for hepatitis B and C, Anti‐HIV and FTA‐ABS) will also be carried out.
Harvest of stem cells from bone marrowPatients will be placed in the prone position and maintained on nasal oxygen by catheter (2l/min). Monitoring during the harvest in the operating room, will include pulse oximetry, blood pressure and electrocardiogram. Local anesthesia with lidocaine 2% will be infused in the lumbar region without a vasoconstrictor. The kit for the collection and filtration of the bone marrow (Baxter Healthcare Corporation™, Valencia, CA) will be used together with 2.5mL of heparin (heparin sodium 500 UI/mL, Roche™; Rio de Janeiro, Rio de Janeiro, Brazil) per 200mL (final volume of bone marrow collected). The harvest will be conducted with a Jamshidi needle (Pharmaseal™; Baxter Healthcare Corporation, Valencia, CA) with 10 punctures on each anterior iliac crest. The bone marrow will be aspirated so that negative pressure will be created to withdraw a larger amount of hematopoietic stem cells. The collected material will be added to the collection bag and subjected to gentle homogenization. The clamps of the kit will be opened and the bone marrow filtered by gravity. A sample (3mL) will be aseptically collected separately to perform blood culture, cell viability and serum antibody (CD34+ and 133+) studies. The clamps will be closed and a volume of 40mL of saline (0.9%) will be added to the collection bag in order to capture the residual volume of the bag and filters and dilute the bone marrow collected to facilitate separation. The clamps must be opened again so that the saline and sediment of the collection bag can be filtered.
Preparation of the stem cellsInside a laminar flow hood, 20mL aliquots of Ficoll‐Hypaque Premium™ (GE Healthcare; Uppsala, Sweden) will be placed in centrifuge tubes of 50mL (polypropylene – Corning™; São Paulo, SP, Brazil) and then 30mL of the bone marrow (diluted 1:1 with saline) will be carefully added to each tube, making a total volume of 50mL inside the tubes. Subsequently, the tubes will be centrifuged at 300×g for 30min at 18°C.
The ring of mononuclear cells formed above the Ficoll layer will be collected with a sterile Pasteur pipette. Next, the mononuclear cells will be transferred to other 50mL centrifuge tubes. The volume must be completed with RPMI culture medium (Cultilab™; Campinas, São Paulo, Brazil). The tubes will be centrifuged at 300×g for 15min at 18°C.
After centrifugation, the supernatant will be discarded. The precipitate will be re‐suspended in RPMI culture medium. The contents of all the tubes will be aggregated and then centrifuged at 300×g and 18°C for 10min. The precipitate will be re‐suspended in 30mL of saline with 5% of albumin and centrifuged at 300×g and 18°C for 10min. Before this step, the cells must be counted in a Neubauer chamber with Turk's solution and trypan blue (Chemistry Dynamics™; São Paulo, São Paulo, Brazil) to estimate cell viability. After this, the precipitate will be re‐suspended to a final volume of approximately 30mL with albuminated saline solution to satisfy two parameters: volume (30mL) and final concentration (1×108 mononuclear cells – BMMC/mL). The total volume will be filtered using Cell Strainer filters (BD Falcon™; San Jose, Califórnia, USA) with a porosity of 100μm to remove any impurities.
Two counts with Turk's solution and trypan blue will be performed: the first after the collection of the bone marrow and the second one after processing the cells. Samples of 5.0mL of the processed bone marrow will be withdrawn for determination of cell viability, immunophenotyping by flow cytometry, performance of blood culture and Hexametil‐propilen‐amino‐oxima (HMPAO™ + 99mTc) staining.
Stem cells infusionIn this protocol, cells from the bone marrow mononuclear cell (BMMC) pool will be infused into a peripheral vein, preferably the medial brachial vein or a similar large vein. The infusion will be completed after preparation, separation, counting and labeling (optional) of the mononuclear cells. The infusion must proceed slowly, with 30mL of the BMMC pool at a concentration of 1×108 mononuclear cells – BMMC/mL administered over approximately 20min.
Stem cell labeling with Tecnetium (99mTc‐HMPAO™) – optionalIt is proposed that Tecnetium HMPAO (GE Healthcare™; Buckinghamshire, United Kingdom) labeled cells be used [as an alternative form] to validate migration of BMMC to the lung. For optimal cell labeling the methodology proposed by the manufacturer of the kit will be followed.
Scintigraphic analysis/nuclear medicine mappingIf Tecnetium HMPAO is used, then nuclear medicine mapping should be performed. With this method, scintigraphic readings over the anterior and posterior thorax can be done 10min to 1h after the injection of labeled mononuclear cells. A whole‐body image should also be obtained to examine the proportion of cells retained in the lungs in relation to the total body count. Before the procedure, the patients will undergo a baseline ventilation and perfusion study.
Patient follow‐up: short, medium and long termIt is essential that patients return for outpatient follow‐up after being discharged from hospital. The following schedule of consultation and outpatient follow‐up is recommended in relation to the procedure date: Visit 1: 7 days, Visit 4: 3 months, Visit 2: 1 month, Visit 5: 6 months, Visit 3: 2 months, Visit 6: 12 months.
The schedule for post‐procedure clinical evaluations and laboratory examinations is summarized in Table 1.
Schedule for post‐procedure clinical evaluations and complementary examinations.
| Clinical and laboratorial parameters | Timing in relation to the procedure date | |||||
| 7 days | 1 month | 2 months | 3 months | 6 months | 12 months | |
| Visit for clinical evaluation | X | X | X | X | X | X |
| Complete spirometry | X | X | X | X | X | |
| Chest X‐ray | X | X | X | |||
| Chest CT | X | |||||
| Scintigraphy: ventilation/perfusion | X | |||||
| Arterial blood gases | X | X | X | |||
| CBC | X | X | X | X | X | X |
| CEA – tumor marker | X | |||||
| 6‐min walking test (Cardiopulmonary Test I) | X | X | X | X | X | |
| Dyspnea Scale Score | X | X | X | X | X | X |
There has been a series of studies using treatment with stem cells in animal models of pulmonary diseases, such as COPD and fibrosis, as well as cell therapy with stem cells in patients suffering from various diseases.9–17
In our laboratory a study was conducted using ASC in mice with experimentally induced COPD. The results showed, in a qualitative and quantitative manner, regeneration of lung tissue in animals with emphysema and they were treated with a pool of BMMC. In this previous work, the presence in the pulmonary tissue of two metalloproteinases (MMP9 and MMP12), which play a key role in the inflammatory process,9 was evaluated. The authors observed that the animals exposed to elastase showed an increased expression of the two metalloproteinases in the lung parenchyma when compared to the control group, showing the role of inflammation in the pathogenesis of emphysema and the role of BMMC, more specifically mesenchymal stem cells, in the modulation of the inflammatory process.9
Several aspects of the cell therapy process have been questioned, such as process optimization, selection and minimal manipulation of cells, autologous versus allogeneic therapy, choosing the best route for application and the infusion of the appropriate amount of cells, as well as the amount and nature of infused cells engrafted in the committed tissue and the action mechanism of these cells on the lung tissue, as stated by Agostini in 2010.21 In addition, the role of the BMMC in both the regenerative process and the pathogenesis of COPD has been the subject of debate, by Caramori et al.20
Despite questions outstanding about the methodological procedures and mechanisms of action, in our previous study we observed that the mice exposed to elastase and submitted to BMMC infusion showed an amelioration of the morphological aspects and decreased concentration of metalloproteinases in the lung tissue.9 Altogether, these data indicate that the cell therapy with BMMC ameliorates COPD condition4,9,11 by modulating inflammation, as also previously discussed by Tzouvelekis et al.22
Alongside these results, pulmonary chimerism in human patients submitted to bone marrow allogeneic transplant was verified.23,24 It is also suggested that the pulmonary microvasculature functions as a barrier where intravenous administered cells are preferentially attracted and retained,23,24 and chronic inflammation in lungs produces molecules that signal and can recruit SC (endogenous and transplanted) with the potential for reconstruction of lung parenchyma.25 The results of these studies provided the experimental foundation, signaled the possibility of application of cell therapy in human patients and substantiated the hypothesis that stem cells would be helpful for the treatment of COPD.26
The experimental protocol proposed in this article refers to the project submitted to the National Committee of Ethics in Research (CONEP), registration N°14.764. This study protocol is also registered with ClinicalTrials.gov (NCT01110252). The original proposal sent to CONEP referred to the execution of a clinical protocol with the profile of a clinical study with the main objective of evaluating the safety of cellular therapy from a BMMC pool in patients with advanced COPD. It is therefore treated as a Phase I clinical protocol.
The procedure was demonstrated to be sufficiently safe. It was tried out in four patients and there were no reports of serious complications among any of the voluntary subjects of this study. The partial results, based on a small sample of only four patients, indicate that cellular therapy with BMMC pools is quite safe and virtually free of significant adverse effects.
Another aspect of this project to be reviewed refers to the use of G‐CSF (granulocyte colony stimulating factor). As the literature thoroughly documents, this drug induces the proliferation and mobilization of hematopoietic cells27–31 and has been widely used in cellular therapy in experimental models and human patients.32–37 In the original protocol, we considered using a daily dose of G‐CSF (5 mcg/kg) for the 3 consecutive days immediately before the marrow puncture and infusion of the BMMC pool. The rationale for using G‐CSF in this study was related to previous results showing that this drug determines an increase in myeloid progenitor cells38 and CD34+25 cells in the bone marrow. Several other protocols, especially the extensive study of cellular therapy in cardiology that is underway in Brazil with the sponsorship of the health department, do not include G‐CSF (EMRTCC. http://terapiacelular.hcl.gov.br/protocols/cardiomyopathiesphp). However, as there is no consensus on the possible advantage of G‐CSF when cells are attained with direct bone marrow puncture,39 G‐CSF can be omitted when a direct bone marrow puncture is adopted.
The results related to stem cell therapy in patients with COPD were first reported in the scientific literature by our team. Apart from the protocol by our research group, two other clinical protocols about cell therapy for COPD are registered on Clinical Trials. The studies are under the responsibility of Osiris Therapeutics Inc. (NTC00683722), already completed, with results recently published,12 and Leiden University Medical Center (NTC01306513). Our results related to autologous cell therapy with pool of BMMC showed that the procedure is safe and there are no adverse effects in human patients with advanced pulmonary emphysema. Still, an improvement in quality of life was reported by treated patients and there was maintenance and/or a discrete improvement in spirometric parameters.11
Recent results sponsored by Osiris Therapeutics Inc. (Columbia, MD, USA) and published by Weiss et al. showed a significant reduction in levels of C‐reactive protein one month after transplantation of mesenchymal stem cells, isolated from bone marrow and cultured in vitro, in patients with pulmonary emphysema. There was no obvious improvement in lung function or quality of life in patients treated with mesenchymal stem cells, named and registered as Prochymal® by Osiris Therapeutic Inc.12
However, we hypothesize that the pool of BMMC, a large panel of progenitor cells with the presence of endothelial, hematopoietic and mesenchymal lineages, could then collaborate to reduce inflammation and improve lung function, maybe through reduction of C‐reactive and/or other inflammatory systemic proteins. All these results and possibilities support the proposition and establishment of this cell therapy protocol for employment in a large number of research centers. As proposed by Tzouvelekis et al., further multicentric randomized studies are needed, in order to establish important issues regarding the safety and efficacy of cell therapy with adult stem cells in lung diseases.26
According to the different aspects discussed in this article, the proposal of this protocol is to establish a safe clinical procedure for therapeutic employment of BMMC for the treatment of patients with chronic obstructive pulmonary disease and, as pointed out above, to maximize the safety conditions of the procedure. In addition, it is an objective of this study that the protocol discussed here may allow the design of subsequent studies to test not only the safety conditions, but also the efficacy of this new methodology.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe Fundação para o Desenvolvimento da Unesp (Fundunesp), Prefeitura Municipal de Assis (SP, Brazil) and CIVAP/Saúde provided financial support. The authors M. Marcelino and T. Stessuk were financially supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior). The author C. Faria was financially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Conflicts of interestThe Fundação para o Desenvolvimento da Unesp (Fundunesp), Prefeitura Municipal de Assis (SP, Brazil) and CIVAP/Saúde provided financial support. The authors M. Marcelino and T. Stessuk were financially supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior). The author C. Faria was financially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
The authors thank the Fundação para o Desenvolvimento da Unesp (Fundunesp), Prefeitura Municipal de Assis (SP, Brazil) and CIVAP/Saúde for the financial support. The authors M. Marcelino and T. Stessuk were financially supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior). The author C. Faria was financially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico).
Please cite this article as: Ribeiro‐Paes JT, Stessuk T, Yonashiro Marcelino M, Arruda de Faria C, Quiqueto Marinelli T, de Oliveira Ribeiro‐Paes MJ. Proposta de um protocolo de terapia celular para o tratamento da doença pulmonar obstrutiva crônica. Rev Port Pneumol. 2014;20:84–91.