In individuals with COPD, reduced exercise capacity is multifactorial, encompassing not only ventilatory limitation but also reduced peripheral muscle function, nutritional status, cardiovascular and other comorbidities.1
Pulmonary rehabilitation programs (PRP) are recognized tools in the comprehensive management of individuals with persistent breathlessness, exercise limitation, and impaired health-related quality of life.2 Inspiratory muscle training (IMT) has been proposed as an additional intervention for PRP. However, multicentre randomized controlled trials (RCTs) have shown that adding IMT to PRP does not improve exercise performance as assessed by the six-minute walking test (6MWT), despite increases in maximal inspiratory pressure (MIP).3–6 The same interest has not been devoted to the potential role of expiratory muscle training (EMT), in fact standard PRP for individuals with COPD does not include any specific program of EMT.2
This study aimed to evaluate expiratory muscles (assessed by maximal expiratory pressure: MEP) compared with inspiratory and peripheral muscles as a determinant of exercise capacity assessed by 6MWT in people with COPD with indications for standard outpatient PRP. If a role in exercise capacity was indicated then there might be potential for performing an RCT to evaluate the addition of EMT to PRP for these individuals.
We performed a post-hoc analysis of data from a larger dataset collected from 134 individuals admitted to outpatient PRP from 2015 to 2019 (EC 2287, May 14, 2019).7 Participants had undergone the evaluation of lung function, MIP, MEP, Maximal voluntary contraction (MVC) of quadriceps and biceps, and 6MWT. The data are shown as absolute values and percentages (%) of predicted.8–12 We studied the association between variables by Pearson's correlation and conducted a multivariate linear regression, employing a stepwise method to identify factors related to the 6MWT. Independent variables included were age, sex, body mass index (BMI), forced expiratory volume at one second (FEV1%), arterial oxygen tension to Inspiratory oxygen fraction (PaO2/FiO2) ratio, Cumulative Illness Rating Scale (CIRS, score), MIP (cmH2O), MEP (cmH2O), MVC-biceps (kg), MVC-quadriceps (kg), and 6MWT (meters). The best model, based on R2, was presented. A significance threshold of p < 0.05 was used.
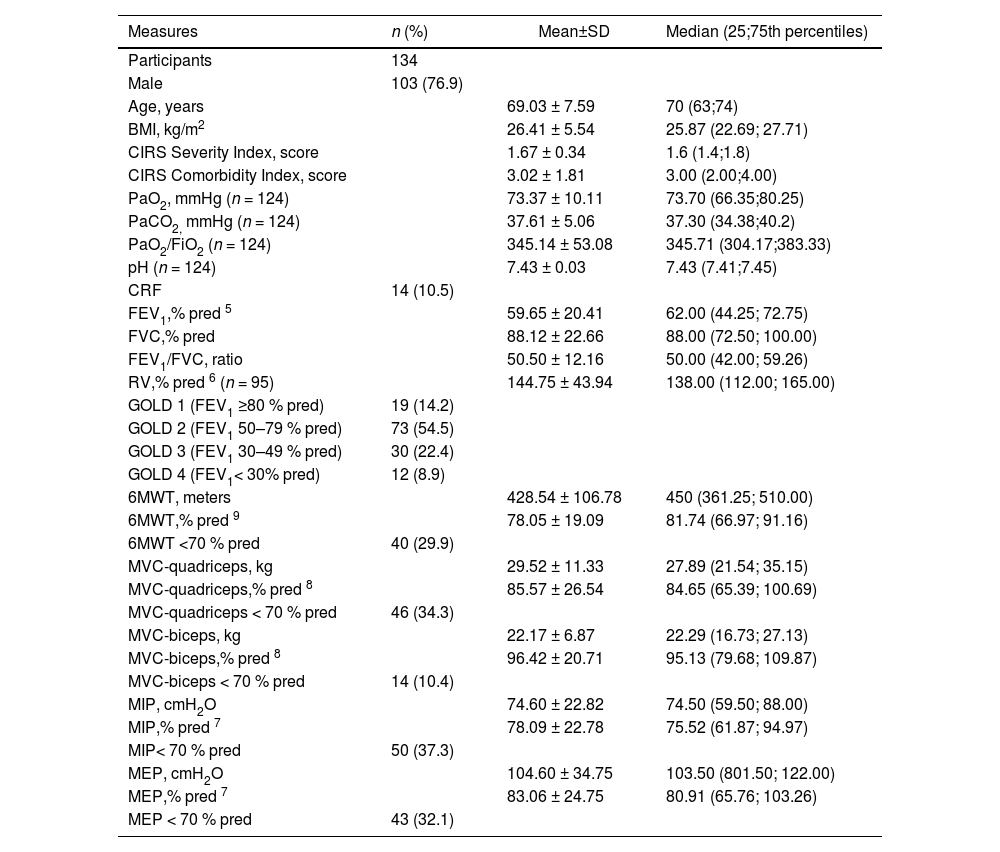
As shown in Table 1, according to airway obstruction, almost 70 % of participants were distributed in GOLD Grades 1 and 2. According to a pre-defined threshold of impairment (<70 % of predicted values)5–9 less than 40 % of participants showed impairment in their respiratory, peripheral muscle force, or exercise capacity.
Anthropometric, demographic, physiological characteristics of participants.
| Measures | n (%) | Mean±SD | Median (25;75th percentiles) |
|---|---|---|---|
| Participants | 134 | ||
| Male | 103 (76.9) | ||
| Age, years | 69.03 ± 7.59 | 70 (63;74) | |
| BMI, kg/m2 | 26.41 ± 5.54 | 25.87 (22.69; 27.71) | |
| CIRS Severity Index, score | 1.67 ± 0.34 | 1.6 (1.4;1.8) | |
| CIRS Comorbidity Index, score | 3.02 ± 1.81 | 3.00 (2.00;4.00) | |
| PaO2, mmHg (n = 124) | 73.37 ± 10.11 | 73.70 (66.35;80.25) | |
| PaCO2, mmHg (n = 124) | 37.61 ± 5.06 | 37.30 (34.38;40.2) | |
| PaO2/FiO2 (n = 124) | 345.14 ± 53.08 | 345.71 (304.17;383.33) | |
| pH (n = 124) | 7.43 ± 0.03 | 7.43 (7.41;7.45) | |
| CRF | 14 (10.5) | ||
| FEV1,% pred 5 | 59.65 ± 20.41 | 62.00 (44.25; 72.75) | |
| FVC,% pred | 88.12 ± 22.66 | 88.00 (72.50; 100.00) | |
| FEV1/FVC, ratio | 50.50 ± 12.16 | 50.00 (42.00; 59.26) | |
| RV,% pred 6 (n = 95) | 144.75 ± 43.94 | 138.00 (112.00; 165.00) | |
| GOLD 1 (FEV1 ≥80 % pred) | 19 (14.2) | ||
| GOLD 2 (FEV1 50–79 % pred) | 73 (54.5) | ||
| GOLD 3 (FEV1 30–49 % pred) | 30 (22.4) | ||
| GOLD 4 (FEV1< 30% pred) | 12 (8.9) | ||
| 6MWT, meters | 428.54 ± 106.78 | 450 (361.25; 510.00) | |
| 6MWT,% pred 9 | 78.05 ± 19.09 | 81.74 (66.97; 91.16) | |
| 6MWT <70 % pred | 40 (29.9) | ||
| MVC-quadriceps, kg | 29.52 ± 11.33 | 27.89 (21.54; 35.15) | |
| MVC-quadriceps,% pred 8 | 85.57 ± 26.54 | 84.65 (65.39; 100.69) | |
| MVC-quadriceps < 70 % pred | 46 (34.3) | ||
| MVC-biceps, kg | 22.17 ± 6.87 | 22.29 (16.73; 27.13) | |
| MVC-biceps,% pred 8 | 96.42 ± 20.71 | 95.13 (79.68; 109.87) | |
| MVC-biceps < 70 % pred | 14 (10.4) | ||
| MIP, cmH2O | 74.60 ± 22.82 | 74.50 (59.50; 88.00) | |
| MIP,% pred 7 | 78.09 ± 22.78 | 75.52 (61.87; 94.97) | |
| MIP< 70 % pred | 50 (37.3) | ||
| MEP, cmH2O | 104.60 ± 34.75 | 103.50 (801.50; 122.00) | |
| MEP,% pred 7 | 83.06 ± 24.75 | 80.91 (65.76; 103.26) | |
| MEP < 70 % pred | 43 (32.1) |
Legend: Data are shown as mean±SD and median (25–75th percentiles), or n (%).
Abbreviations: 6MWT: Six-minute walking distance test; BMI: Body-mass Index; CIRS: Cumulative Illness Rating Scale; CRF: Chronic respiratory failure; FEV1: Forced Expiratory Volume at 1 s; FVC: Forced Vital Capacity; MVC: Maximal Voluntary Contraction; MEP: Maximal Expiratory Pressure; MIP: Maximal Inspiratory pressure; PaCO2: Arterial carbon dioxide tension PaO2: Arterial oxygen tension; RV: Residual Volume.
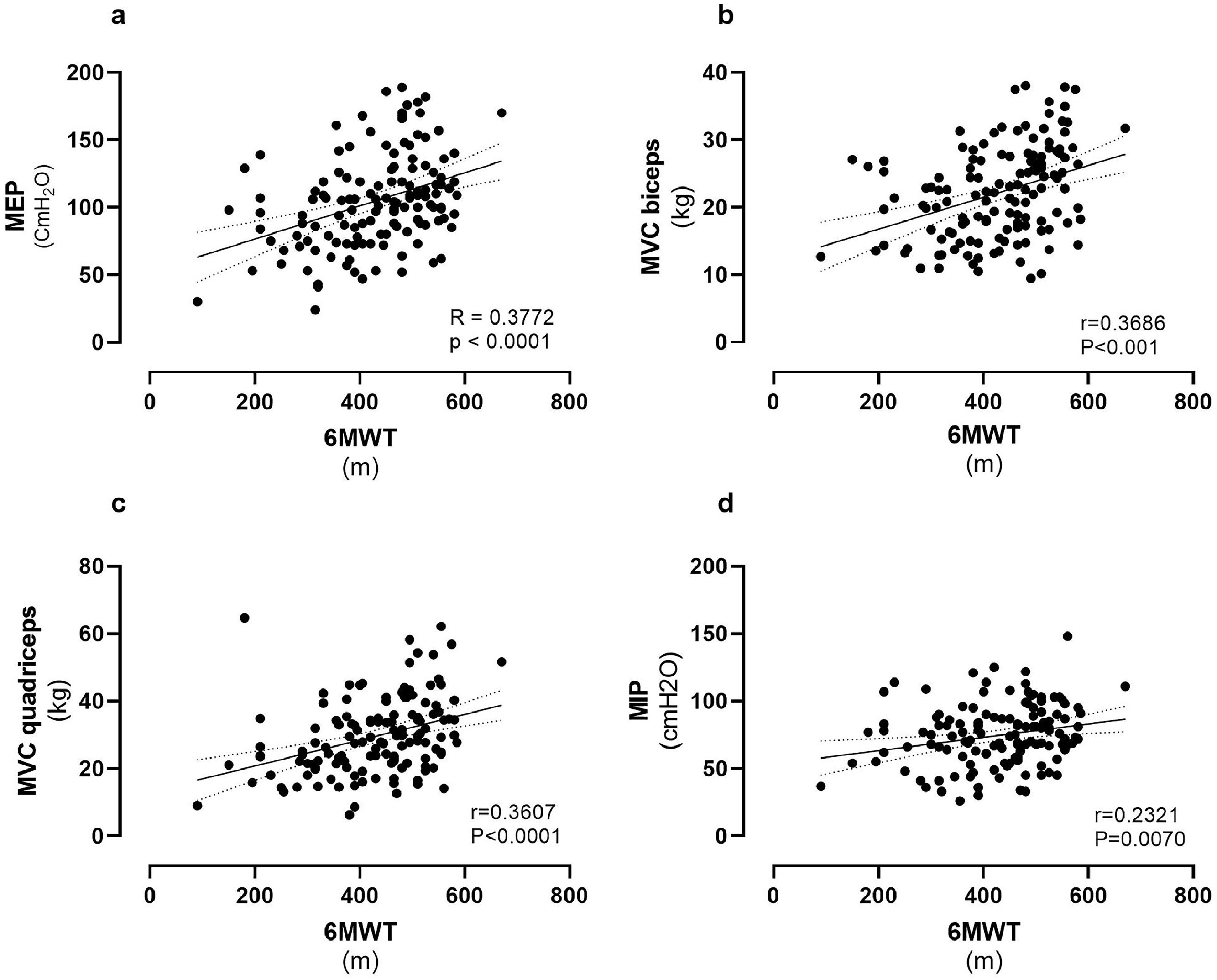
Fig. 1 shows the correlation between 6MWT and muscle force using absolute values. The strongest, although weak, correlation was found for MEP, whereas MIP showed the weakest correlation.
When considering % of predicted values,5–9 MIP showed no significant correlation with 6MWT (R = 0.0098, P = 0.9103). The correlations of MEP (R = 0.2747, P = 0.001), quadriceps, and biceps MVC (R = 0.2725, P = 0.0015 and R = 0.2504, P = 0.0035 respectively) with 6MWT were similar but weaker than when assessed using absolute values. The multivariate regression model (R2= 0.3828) showed 6MWT (meters) = 416.70+2.2353 (Bstandard (Bstd) 0.2371) × MVC-quadriceps + 0.8073 (Bstd 0.2627) × MEP + 1.8053 (Bstd 0.3449) × FEV1 - 89.8741 (Bstd −0.2612) X CIRSSeverity Index– 4.1009 (Bstd −0.2127) X BMI.
The role of the respiratory muscles at rest and during exercise has been extensively studied. Unlike at rest, during exercise, the diaphragm, the main inspiratory muscle, is primarily a “flow generator”, meaning that its mechanical output results mainly in velocity of shortening rather than pressure.13 The abdominal muscles act on the abdomen and abdominal rib cage and are expiratory.
While the finding of significant correlations of airway obstruction and quadriceps MVC (one of the main muscle groups involved in walking) with 6MWT is not surprising, the strongest correlation of MEP and the weak or absent correlation of MIP with 6MWT are the original findings of our study. These results may lead to some considerations for PRP.
First, the accepted standard PRP includes different modalities of leg exercise training with undisputed success.2,14 The positive correlation between quadriceps MVC and 6MWT must be considered with caution when considering the success of leg training in improving 6MWT. It has not been convincingly demonstrated so far that increasing leg muscle strength with a specific intervention during PR (i.e. lower limb resistance training) will result in greater improvements in 6MWT.15
Second, the weakest correlation between MIP and 6MWT may be one explanation for the failure in improving 6MWT of well-conducted RCT studies regarding IMT, although all trials were able (with different success rates) to increase MIP.3–5 Apart from the physiological reasons reported elsewhere,6 what is the rationale of training muscles such as the inspiratory ones, which are not (or shardly) involved in the assessed outcome? However, this issue is far from a final definition, and further studies are required.16
Thirdly, although EMT has been suggested as a tool to improve effective cough in diseases and conditions other than COPD, unfortunately, to the best of our knowledge no high-quality RCT on the effect of EMT on exercise capacity has been conducted in COPD populations so far.17 The strongest correlation of MEP with 6MWT may suggest the need for future RCTs investigating the effects of EMT on exercise capacity in individuals with COPD, using appropriate techniques (such as active “core” muscle training, abdominal muscles electrostimulation, or using dedicated breathing devices). However, the overall weakness of correlations confirms the multifactorial influence on exercise capacity and it can be argued that this may also forecast an unsuccessful outcome for EMT alone. In addition, the physiological basis for EMT may be controversial. Unlike at rest, the expiratory muscles play an active role in breathing during exercise. 13 While it is possible that increased intra-abdominal pressures during exercise may help to improve diaphragmatic function, studies have shown that this increased expiratory muscle activity does not attenuate the increase in end-expiratory lung volume (EELV), the relatively early mechanical limitation of ventilation and the associated dyspnoea.18 Therefore, interventions aimed at improving expiratory muscle function may not be as useful as assumed. However, these theoretical considerations should not prevent further RCTs.
To avoid any bias associated with anthropometrics and demographics, we also tested the correlations using % of predicted values, which showed similar hierarchies and directions but were weaker than when using absolute values. Anthropometric, and demographic characteristics, may influence PRP success.19
Our participants had mild to moderate levels of static hyperinflation as assessed by residual volume. It remains to be elucidated, whether the results of the present study will be found also in individuals with more severe static hyperinflation. In people with COPD, static hyperinflation may influence exercise capacity as assessed by the 6MWT.19 However, dynamic hyperinflation (DH) was not assessed in our participants and we cannot confirm previous findings of DH as a potential mechanism of reduced physical activity in non-severe COPD without cardio-vascular comorbidities such as our participants.20
The main limitations of our study are related to its retrospective, single-centre design which resulted in some missing data or lack of important physiological measures such as DH or level of dyspnoea during the 6MWT. However, in addition to the relatively new approach to analysis, our study represents a real-life condition at a time when even RCTs are being questioned.21
Among the accepted outcome measures of PRP only 6MWT was assessed: whether our results also apply to other outcomes such as dyspnoea should be specifically evaluated. Our results apply mainly to people with mild to moderate COPD.
In conclusion, with the limitations mentioned above, our study shows that in individuals with COPD, expiratory muscle force as assessed by MEP has the strongest correlation with exercise capacity as assessed by 6MWT, whereas inspiratory muscle force assessed by MIP has the weakest correlation. Although the overall weak correlations do not predict successful results and physiological bases for EMT are questionable, our findings may suggest the need for RCT to evaluate the effectiveness of EMT in improving the exercise capacity of these individuals. It does not seem reasonable to exclude any muscle group a priori from these future studies in order to provide the much-needed evidence to find out which individuals might benefit from adding these specific exercises to their PR program. The current lack of evidence for EMT (and IMT) should not by any means be confused with evidence of absence of any potential effect. The history of research is full of unexpected results, isn't it?
The authors thank Laura Comini and Adriana Olivares for their technical assistance. This work was supported by the “Ricerca Corrente” Funding scheme of the Ministry of Health, Italy.